Targeting the Epidermal Growth Factor Receptor
The epidermal growth factor receptor (EGFR) promotes the growth of different cell types and has been implicated in tumorigenesis. The EGFR comprises a family of four structurally similar tyrosine kinases with a complex link to downstream signaling molecules that ultimately regulate key cell processes. Anti-EGFR agents have been developed as promising therapeutic anticancer targets, and some have been recently approved for the treatment of non-small-cell lung cancer and colon cancer. The two anti-EGFR therapies with the greatest clinical application are monoclonal antibodies that block the binding of ligands to EGFR and small-molecule tyrosine kinase inhibitors that inhibit the binding of adenosine triphosphate to the internal tyrosine kinase receptor of EGFR. We attempt to give an overview of the EGFR function and biology, focusing on the most important clinical findings and applications of EGFR inhibitors in lung and head and neck cancer.
The epidermal growth factor receptor (EGFR) promotes the growth of different cell types and has been implicated in tumorigenesis. The EGFR comprises a family of four structurally similar tyrosine kinases with a complex link to downstream signaling molecules that ultimately regulate key cell processes. Anti-EGFR agents have been developed as promising therapeutic anticancer targets, and some have been recently approved for the treatment of non-small-cell lung cancer and colon cancer. The two anti-EGFR therapies with the greatest clinical application are monoclonal antibodies that block the binding of ligands to EGFR and small-molecule tyrosine kinase inhibitors that inhibit the binding of adenosine triphosphate to the internal tyrosine kinase receptor of EGFR. We attempt to give an overview of the EGFR function and biology, focusing on the most important clinical findings and applications of EGFR inhibitors in lung and head and neck cancer.
The epidermal growth factor receptor (EGFR) is a 170-kDa membrane-anchored protein tyrosine kinase that has been implicated in tumorigenesis. Protein kinases are targets for the treatment of numerous diseases including cancer, inflammatory disorders, and diabetes. There are 518 protein kinases that have a shared catalytic domain as far as structure, yet the regulation of their catalysis is variable.[1]
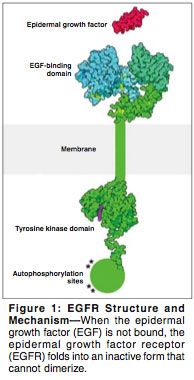
The receptors in this family (ErbB/HER) consist of four tyrosine kinases that are structurally similar and include (EGFR; HER1 or ErbB-1), HER2/neu or ErbB-2, HER3 or ErbB-3, and HER4 or ErbB-4. All share an extracellular domain, an intracellular tyrosine kinase, and a transmembrane domain. Ligands such as transforming growth factor (TGF)-alpha or epidermal growth factor (EGF) activate the EGFR, resulting in its dimerization or heterodimerization with other receptors that are closely related, such as HER2/neu. Phosphorylation of these receptors through their tyrosine kinase domains leads to the recruitment of downstream effectors and activation of proliferation and cell-survival signals (Figure 1).[2] This process appears to be overactive in malignancy.[3] An important signaling route of the ErbB family is the Ras-Raf-MAP-kinase pathway. Through activation of Ras, a multistep cascade of phosphorylation is initiated; this leads to activation of the MAPKs ERK1 and ERK2,[4,5] both of which are linked to cell proliferation, transformation, and survival in laboratory studies.
We now have a better understanding of the structure of EGFR as well as mutations encountered in human cells because of data from the human genome project.[6] It is of note that EGFR signaling may be affected by mechanisms other than EGFR expression.[7] Mutation in the EGFR has been observed in a variety of tumors. The EGFRvIII mutant lacks the external ligand-binding domain but has a tyrosine kinase that is constitutively activated.[8]
The ErbB network is key to important signaling pathways and has been preserved throughout evolution. Research in knockout and transgenic mice has clarified some of its important functions. Inactivation of the ErbB1 subtype seems to impair epithelial development such as tooth growth and eye opening.[9,10] ErbB1 seems to play an important role in differentiation of epithelial components of skin, lung, pancreas, and gastrointestinal tract. Mice that lack expression of TGF-alpha (an EGF ligand), have abnormalities in eye, skin, and hair development.[11]
Although expressed in nonmalignant cells, the EGFR is highly expressed in a variety of tumors, and its expression is correlated with poor response to treatment and poor survival.[12] Its activation has been shown to promote tumor proliferation, angiogenesis, as well as metastases and invasion.[8,13] It was earlier suggested that abnormal EGFR signaling is correlated with advanced disease, poor response to chemotherapy, and poor prognosis.[14,15] Human malignancies use several mechanisms that activate the EGF pathway, including overproduction of ligands, overproduction of receptors, or constitutive activation of receptors.[16,17] More recent data, however, indicate that overexpression of EGFR in non-small-cell lung cancer (NSCLC) may not be correlated with poor outcome.[18] EGFR is overexpressed in 40% to 80% of NSCLC cases and numerous other epithelial cancers.[19]
EGFR Biology
Similar chemical characteristics of EGFR in humans and mice indicate its preservation throughout a long evolutionary process.[20] Radioimmunoassays have shown that EGFR is present in various body fluids including urine, saliva, amniotic fluid, and plasma.[21] EGF has been shown to promote the growth of different cell types in tissue cultures including fibroblasts, mammary epithelium, and vascular endothelium.[22]
The receptor is composed of a single chain with an outer portion that forms an EGF-binding domain (Figure 1). When the multipart portion of the receptor binds to EGF, it changes shape, allowing the receptor to dimerize with other receptors. The inner part of the receptor, which is a tyrosine kinase enzyme, is activated upon dimerization and adds phosphate groups to the tyrosine residues. This initiates a signaling cascade intracellularly that ultimately promotes DNA synthesis and cell growth. The growing information about the structure and function of this receptor has promoted the development and discovery of new anticancer drugs blocking the action of EGFR from both ends.
The potency of intracellular signaling seems to be determined by the ligand and which intracellular sites are autophosphorylated. The PI3K-activated AKT pathway and P70S6/P85S6K have different activation potencies that probably depend on the type of receptor (ErbB1/2/3/4).[23] Several pathways including the MAPK pathway, the stress-activated protein kinase pathway, protein kinase C, and Akt are simultaneously activated. These lead to activation of different transcription programs in the nucleus.[23] The result is activation of cell division and migration, both of which are characteristics of tumor cells. There appear to be other signaling pathways that are also integrated into the ErbB network including hormonal pathways, neurotransmitters, lymphokines, and stress inducers (Figure 1).[24]
Tyrosine kinase inhibitors (TKIs) have been studied extensively in tissue culture of transformed cells and in animal models. They have been shown to inhibit receptor phosphorylation as well as tumor growth, invasion, and adhesion.[25] They have also been shown to reverse cancer cells to phenotypically differentiated cells and hence reverse the process of transformation.[26] Some quinazoline derivatives have been shown not only to compete with adenosine triphosphate but also to promote the formation of EGFR dimers that are inactive, and in the absence of ligands. Whether this property in these particular agents has any clinical implications is not yet known.[27]
Preclinical Validation
Monoclonal antibodies to EGFR, such as cetuximab (IMC-C225, Erbitux), have been shown to inhibit cell growth in vitro and in vivo.[28] They have also been shown to inhibit apoptosis as well as metastases in cell lines.[29,30] TKIs such as gefitinib (ZD 1839, Iressa) have been shown to inhibit cell proliferation and induce apoptosis in addition to having antiangiogenic properties.[31] Moreover, gefitinib was shown to inhibit metastases and cell migration by blocking p21-activated kinase 1, and has been shown to inhibit metastases in head and neck and breast cancer cells.[32]
Preclinical studies showed these targeted agents to potentiate the effect of cytotoxic chemotherapy. Cetuximab can enhance the effects of chemotherapeutic agents as well as radiation therapy.[33,34] In combination with topoisomerase I inhibitors, cetuximab was shown to improve survival of nude mice with human colon cancer xenografts when it was sequentially used with topotecan (Hycamtin).[35] Similar data exist for gefitinib, which has been shown to potentiate cytotoxic drug activity on cancer cells as well as to act synergistically with radiation.[36,37]
Clinical Experience
Following models established in preclinical trials, EGFR inhibitors were developed for combination with chemotherapy or radiation. Among the monoclonal antibodies, cetuximab and panitumumab have been developed most extensively at the clinical level.
Several TKIs are under investigation; the two most extensively studied TKIs in clinical trials are gefitinib and erlotinib (OSI-774, Tarceva). Gefitinib is active in multiple tumor cell lines including lung, colorectal, and ovarian tumors.[36] Preclinical studies of erlotinib have shown a significant dose-dependant inhibition of head and neck tumor cell lines.[38] The fact that EGFR/HER1 can form heterodimers with other HER receptors, has prompted the attempt to develop pan-HER inhibitors. CI-1033 is one such inhibitor, which targets all four EGFR/HER receptors without significant interaction with other tyrosine kinase receptors.[39] Other multitargeting EGFR inhibitors include GW572016, which in preclinical studies reduced TK phosphorylation and induced apoptosis in HN5 cell lines. It also inhibited the activation of downstream molecules such as AKT and Erk 1 and 2 involved in regulating proliferation and cell survival.[40]
Skin rash is the most common adverse reaction associated with anti-EGFR therapy. Skin rash has been noted to be a surrogate marker for response of tumors to EGFR inhibitors. The reason for this correlation may be related to the high levels of EGFR expression in dermal epithelium and the likelihood that EGFR inhibitors may exert the same biologic effect on skin as on tumor tissue.[41]
It is worth mentioning that anti-EGFR therapy has been applied to a multitude of tumors besides head and neck and lung cancers (the topic of this discussion). Based on phase II clinical trials, the anti-EGFR monoclonal antibody cetuximab was approved as a second-line therapy for patients with colorectal carcinoma refractory to irinotecan (Camptosar). In one study, adding cetuximab to irinotecan in patients with irinotecan-refractory metastatic colorectal cancer resulted in a longer time to progression, a higher relative risk, and a better overall survival than seen with cetuximab alone.[42] In another study, cetuximab monotherapy was evaluated in patients with irinotecan-refractory colorectal cancer overexpressing EGFR. Nine percent of patients achieved a partial response.[43] Cetuximab has not been tested in patients for whom oxaliplatin (Eloxatin)-based regimens have failed.
Currently, two phase III trials are in progress. One trial is enrolling patients with EGFR-positive metastatic colorectal cancer in whom irinotecan failed, and who are being randomized to get FOLFOX4 (5-FU/leucovorin/oxaliplatin) or FOLFOX4 plus cetuximab. Safety data were presented at the 2004 meeting of the American Society of Clinical Oncology (ASCO).[44] The Cancer and Leukemia Group B (CALGB) is also evaluating cetuximab in the first-line setting.[45] In addition, the gastrointestinal intergroup is conducting a phase III trial examining the efficacy of cetuximab with various chemotherapy regimens in the adjuvant setting.[46]
Head and Neck Cancer
The humanized version of cetuximab, EMD 72000, has been developed for clinical use after the original mouse monoclonal antibody was associated with anaphylactic reactions as well as loss of efficacy after repeated exposures. The humanized version of cetuximab has had promising efficacy in phase I and II trials.[47] In a phase IB study of cetuximab in combination with cisplatin in patients with recurrent squamous cell carcinoma of the head and neck (SCCHN), infused cetuximab was noted to bind and saturate tumor EGFR receptor, justifying it as a potential novel antitumor agent for patients whose cancers express EGFR.[48] Cetuximab is currently in clinical phase II/III trials in several tumor types including colorectal,[29] pancreatic,[49] prostate,[50] and squamous cell carcinoma of the head and neck.[33]
• Early Trials-Pilot studies have reported a good tolerability of cetuximab in combination with radiation and chemotherapy in patients with SCCHN.[51] Results of initial phase II studies in head and neck squamous cell cancer have been applied to patients with chemorefractory disease (Table 1).[52-60] In a trial reported by Kies et al, patients received identical chemotherapy plus cetuximab. The researchers noted a 12% response rate for the combination regimen in the 78 patients enrolled.[52] In a second trial, patients were allowed to receive two to four cycles of cisplatin or carboplatin, and after disease progression, receive the same chemotherapy regimen with cetuximab. The reported response rate was similar (11%),[53] suggesting some activity of cetuximab in chemo-refractory disease.
Results from a phase II trial of cetuximab in platinum-refractory SCCHN were reported at the 2004 ASCO annual meeting. A total of 103 patients with stage III/IV recurrent or metastatic SCCHN with documented progression on a platinum regimen were treated with cetuximab until disease progression. An overall response rate of 12.6% was reported.[54] A recent phase I study combined cetuximab with cisplatin/carboplatin and fluorouracil (5-FU) in patients with recurrent and/or metastatic SCCHN. An overall response rate of 48% was reported, with the most frequent side effect being neutropenia (36%).[61]
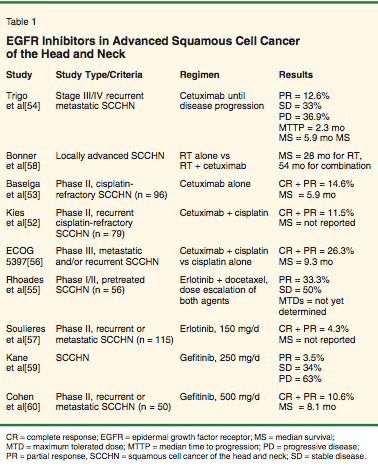
• Phase III Trial-A recent phase III trial investigated cetuximab in combination with cisplatin vs cisplatin and placebo in patients with metastatic recurrent SCCHN. The study enrolled 116 patients who were stratified by performance status and whether they had recurrent or newly diagnosed disease. Cetuximab produced a modest improvement in median time to progression and median overall survival that did not reach statistical significance (P = .27 and P = .18, respectively). However, there was a superior overall response rate in the cetuximab arm (22.6% vs 9.3%).[56] The lack of statistical significance for the primary end points could have resulted from the fact that the study was underpowered. Another possible explanation is the higher-than-anticipated progression-free and overall survival seen in the control group. ]
• Radiation Therapy Combinations-Cetuximab was also combined with radiation therapy in different trials, as a single agent or in combination with cisplatin. In a phase I trial enrolling 16 patients, cetuximab did not seem to enhance the toxicity of radiation therapy in the single-fractionated or hyperfractionated modalities.[62] In another phase I trial in 21 patients, cetuximab was used weekly with cisplatin during weeks 1 and 4 with concomitant boost radiation therapy. A 2-year progression-free survival of 76% was observed. Reported toxic effects included grade 4 anaphylaxis, myocardial infarction, and two deaths.
Another recent phase III trial in SCCHN enrolled 424 patients from different countries who were randomized to 6 or 7 weeks of radiation therapy, vs radiation and weekly cetuximab. The study revealed a prolonged median survival time of 54 months in patients treated with cetuximab/radiotherapy compared to 28 months for patients treated with radiotherapy alone (P = .02). The addition of cetuximab was also associated with a statistically significant improvement in 2- and 3-year survival.[58] A prolonged median survival was reached in patients treated with the combination of cetuximab and radiation (54 months) vs radiation alone (28 months). The 2- and 3-year survival rates were 62% and 57% for patients on the combination arm vs 55% and 44% on the radiation therapy-alone arm (P = .02). The investigators found no significant added toxicity with cetuximab.[58]
Other EGFR-targeted antibodies appear to enhance the effect of radiation therapy in SCCHN and are currently being tested in phase II trials.[63] A phase I trial in patients with SCCHN using h-R3 (a humanized IgG1 anti-EGFR monoclonal antibody) in combination with radiation therapy showed a complete response rate of 50% (11/22) in patients with locally advanced SCCHN. Currently, this strategy is being further investigated in a randomized phase II trial.
• Tyrosine Kinase Inhibitors-TKIs have also entered clinical trials in squamous cell cancer of the head and neck; both gefitinib and erlotinib have been studied in this setting. In a phase II trial, gefitinib was administered at a dose of 500 mg/d to 47 patients, for a reported response rate of 10.6% and a median survival of 8.1 months, with the main toxicity being grade 1/2 dermatologic effects, in addition to diarrhea or nausea and vomiting.[64] Smaller doses were demonstrated to have an equivalent effect; one trial reported a 3% response rate and lower toxicity when using a 250-mg/d dose of gefitinib.[60] The results were updated at the ASCO 2003 meeting.[59] Phase I trials have also combined gefitinib with radiation alone or radiation and chemotherapy.[65]
Erlotinib has been tested in a phase II study of recurrent metastatic SCCHN, for a reported 4% response rate and a median survival of 6 months.[66] Other phase I trials combining erlotinib with cisplatin chemotherapy demonstrated tolerability of the combination. In one phase I/II trial reported at the ASCO 2004 meeting, a maximum tolerated dose of daily erlotinib and weekly docetaxel was determined. The observation of some dose-limiting toxicities including rash, neutropenia and sepsis, and stomatitis suggested that the maximum tolerated dose for the combination is lower than for single-agent therapy.[55] In a phase II study of advanced SCCHN, erlotinib was evaluated in 115 patients at a dose of 150 mg/d. Patients had no more than one prior therapy for advanced disease. Rash was reported in 79% of patients, and the partial response rate was 4.3%, with 38.3% achieving stable disease, suggesting activity and tolerability of erlotinib in this group of refractory patients.[57] Additional phase II trials are under way.[67]
Lung Cancer
• Cetuximab-Several studies are combining cetuximab with chemotherapy in NSCLC.[68] Cetuximab was investigated in NSCLC and preliminary results indicate that cetuximab may improve the efficacy of chemotherapy in first-line treatment of NSCLC. Eighty-six patients were randomized to receive cetuximab with cisplatin and vinorelbine or cisplatin and vinorelbine alone. Patients on the chemotherapy-only arm had a lower overall response rate (20% vs 31.7%) and a shorter time to disease progression (4.2 vs 4.7 months).[69] Another phase II trial evaluated the efficacy of cetuximab combined with docetaxel in patients with lung cancer refractory to first-line therapy. Of 47 evaluable patients, 13 had a partial response.[70]
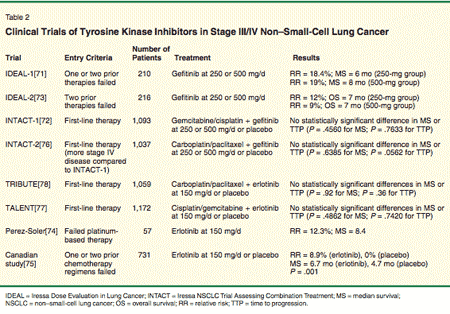
• Gefitinib-EGFR-TKIs have been shown to have clinical activity in solid tumors, with the most common side effect being rash and diarrhea. Phase II trials have demonstrated the efficacy of gefitinib in patients with NSCLC.[71] In the Iressa Dose Evaluation in Lung Cancer (IDEAL) trial (Table 2),[71-78] the efficacy and tolerability of two doses of gefitinib were examined. More than 40% of patients had some response or stable disease, demonstrating that gefitinib is an active and tolerated agent for the treatment of chemotherapy-resistant NSCLC,[71] and is now approved for patients with metastatic NSCLC who have previously received chemotherapy and experience disease progression.
Combining gefitinib with chemotherapy was attempted in two large phase III trials (Table 1): Iressa NSCLC Trial Assessing Combination Treatment (INTACT)-1 and INTACT-2. INTACT-1 enrolled 1,093 patients with unresecable NSCLC who were not treated with chemotherapy. The randomization was between gemcitabine (Gemzar) and cisplatin every 3 weeks with either gefitinib (500 or 250 mg/d) or placebo. Unfortunately, no statistically significant improvement in the primary end point (survival) or time to progression was noted.[72]
• Erlotinib-Another TKI that has reached clinical application in lung cancer is erlotinib. The drug demonstrated clear preclinical activity and was shown to have a higher potency against EGFR/HER1 tyrosine kinase compared to other kinases. It has also been shown to inhibit downstream pathways including MAP kinase and Akt pathways.
Clinically, in phase I trials, diarrhea and skin rash were the main toxicities.[79] In a phase II study, a dose of 150 mg/d was administered to 57 patients with advanced NSCLC who failed prior platinum-based therapy. Twenty-two patients were reported to have stable disease, with an overall response rate of 12.3%.[74]
In a Canadian phase III study of stage IIIB/IV NSCLC patients in whom one or two prior chemotherapy regimens had failed, a total of 731 patients were randomized to 150 mg/d of erlotinib or placebo. Median survival for patients treated with erlotinib was 6.7 months compared to 4.7 months for the placebo group. Progression-free survival was also higher for the treatment arm at 2.2 months compared to 1.8 months for placebo (P < .001). The time period in which symptoms developed was also longer for the erlotinib group. In a subset analysis, patients who had never smoked were noted to derive the most benefit compared to previous or current smokers (P = .03). These findings led to the approval of erlotinib (on November 18, 2004) for use in NSCLC when one or two prior chemotherapy regimens have failed.[75]
Erlotinib has also been tested in combination with cytotoxic chemotherapy in the TRIBUTE study, which combined erlotinib with paclitaxel and carboplatin chemotherapy. In this study, 1,059 patients were randomized between erlotinib and placebo. Unfortunately, as with the INTACT trials, no significant differences were observed in response rates (21.5% for erlotinib vs 19.3% for the control group, P = .36) or in overall survival (10.8 vs 10.6 months). In subset analysis, however, "never-smokers" had a significantly better survival when they were treated with erlotinib (22.5 vs 10.1 months, P = .01).[80]
In the TALENT trial, erlotinib was combined with cisplatin and gemcitabine and compared with placebo added to these two cytotoxic agents. The study enrolled 1,172 patients, but no significant differences in overall survival were noted.[77]
• Patient Selection-Perhaps the most obvious explanation for the failure of these trials to show a survival benefit is that patients were not properly selected. If gefitinib or erlotinib targets the tyrosine kinase of the EGF receptor, then determining the presence of EGFR seems to be a prerequisite. No such preselection of patients was done in the INTACT, TRIBUTE, or TALENT trials. There is also no clear agreement regarding the best means of determining EGFR expression. Measurement of EGFR is not equivalent to measuring the inhibition of the tyrosine kinase, which is the target for TKIs. Recent studies have suggested that even patients with EGFR-negative tumors may still respond to small-molecule TKIs.[81]
Thus, there is currently no molecular marker that would predict who among lung cancer patients would benefit from targeted therapy with gefitinib or erlotinib. Another possible explanation could be that both gefitinib and erlotinib were more effective in previously treated NSCLC rather than chemotherapy-naive patients. Early in the treatment, EGFR and its ligands may be less critical for cancer cell survival. With more exposure to cytotoxic therapy, cancer cells may become more dependant on EGFR.
Multitargeted TKIs have reached clinical trials as well and were tested in phase I studies. CI-1033 was tested in a phase I trial using nine different dose levels. Reported side effects included diarrhea and rash as well as grade III thrombocytopenia in three patients.[82] In another study, intravenous dosing has produced an increase in the bioavailability of CI-1033 without increasing adverse reactions.[83]
Sensitivity of EGFR to Inhibitors
Most patients with NSCLC do not have a clinical response to the EGFR tyrosine kinase inhibitor gefitinib, and initial clinical studies of the drug reported a response rate of only 10% to 19%.[73] Although gefitinib inhibits the growth of cancer-derived cell lines, its effect does not seem to correlate with the level of expression of EGFR.[84] Despite this information, dramatic responses have been observed in some patients; this leads to questions about the mechanism of such a response in selected patients.
For example, it was noticed in the IDEAL-1 trial that patients with adenocarcinoma were three times more likely to respond to gefitinib compared to other histologic types. Female patients also seemed to have a higher response rate compared to males.[71] Lynch et al analyzed tumor samples of patients with NSCLC treated with gefitinib. These investigators conducted a nucleoside-sequence analysis looking for mutations in the EGFR coding sequence. Tumor specimens were analyzed from nine patients who were deemed to have had a significant response. In eight of these nine patients, heterozygous mutations were observed, all of which clustered within the tyrosine kinase domain of EGFR. When the entire coding region of the gene was sequenced in 25 patients with NSCLC not treated with gefitinib, mutations were detected in only two patients with bronchoalveolar carcinomas, suggesting that only a subgroup of cancers in which EGFR signaling plays a role have a detected mutation.[85]
Other studies have shown that somatic mutations in the tyrosine kinase domain of EGFR predict response to gefitinib. Those mutations were more commonly observed in adenocarcinomas as well as in women and patients of Japanese origin.[86] Similar mutations were reported in patients shown to respond to erlotinib, with descriptions of novel mutations compared to those described with gefitinib. Two patients with mutations also showed progression of disease on erlotinib.[87]
Another recent observation suggested a better response to gefitinib in patients with the papillary subtype of adenocarcinoma[88]; this raised the possibility of a benefit from gefitinib in the postoperative treatment of this subtype. Interestingly, both a never-smoker status and diffuse pulmonary metastases are frequently observed in the micropapillary pattern of adenocarcinoma, which has a poor prognosis.[89]
In an analysis of 228 patients from the TRIBUTE study, tissue blocks were tested for mutations. Both survival and time to progression were significantly better among patients with mutant EGFR. This finding was independent of the therapy received.[90]
Skin rash has also been used as a marker for clinical activity, given observations that a rash is a predictor of survival in patients treated with TKIs including erlotinib. Confirmatory phase II trials are ongoing to clarify the relationship between skin rash and response to these agents.[91]
Conclusions
EGFR is highly expressed in a variety of solid tumors, including those of the upper aerodigestive tract, and is associated with poor response to treatment and poor survival. Successful anticancer therapies have been developed to target ErbB-receptor signaling. The exploitation of the ErbB pathway to treat malignancies as well as nonmalignant conditions is still in its infancy. It seems clear from the clinical information accumulated so far that the most important consideration for a successful therapy design is the choice of a suitable target.
Understanding the molecular basis of response to these targeted agents in certain instances and the lack of response in others will have an immediate effect on clinical applications. A good example is the dramatic response to gefitinib observed in patients whose tumors have EGFR mutations. This should open the door for genotype-directed clinical applications of gefitinib and perhaps other TKIs in NSCLC. The relative nontoxic nature of these targeted agents should also raise the question of their potential use in earlier-stage disease as adjuvant therapies or even as possible chemopreventive agents in the future.
A broad spectrum of small-molecule TKIs is being studied. The ability of these agents to inhibit multiple EGFR/HER members may potentially inhibit downstream signaling more effectively. Future research may also focus on combining targeted agents such as two EGFR-targeted agents or combining an anti-EGFR agent with other targeted strategies. Despite all these prospects, EGFR inhibition is still in the early stages of clinical development.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Manning G, Whyte DB, Martinez R, et al: The protein kinase complement of the human genome. Science 298:1912-1934, 2002.
2. Jorissen RN, Walker F, Pouliot N, et al: Epidermal growth factor receptors: Mechanisms of activation and signaling. Exp Cell Res 284:31-53, 2003.
3. Noble MEM, Endicott JA, Johnson LN: Protein kinase inhibitors: Insights into drug design from structure. Science 303:1800-1804, 2004.
4. Alroy I, Yarden Y: The Erb signaling network in embryogenesis and oncogenesis: Signal diversification through combinational ligand-receptor interactions. FEBS Lett 410:83-86, 1997.
5. Lewis TS, Shapiro PS, Ahn NG: Signal transduction through MAP kinase cascades. Adv Cancer Res 74:49-139, 1998.
6. Ciesielski MJ, Fenstermarker RA: Structure of the epidermal growth factor receptor gene and intron recombination in human gliomas. Curr Genom 4:1-12, 2003.
7. Arteaga C: Epidermal growth factor receptor dependence in human tumors: More than just expression? Oncologist 7:31-39, 2002.
8. Wells A: The epidermal growth factor receptor (EGFR)-a new target in cancer therapy. Signal 1:4-11, 2000.
9. Miettinen PJ, Berger JE, Meneses J, et al: Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376:337-341, 1995.
10. Threadgill DW: Targeted disruption of mouse EGF receptor: Effect of genetic background on mutant phenotype. Science 269:230-234, 1995.
11. Mann GB, Fowler KJ, Gabriel A, et al: Mice with null mutations of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell 73:249-261, 1993.
12. Brabender J, Danenberg KD, Metzger R, et al: Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer is correlated with survival. Clin Cancer Res 7:1850-1855, 2001.
13. Woodburn JR: The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther 82:241-250, 1999.
14. Chen Z, Ke LD, Yuan XH, et al: Correlation of cisplatin sensitivity with differential alteration of EGFR expression in head and neck cancer cells. Anticancer Res 20:899-902, 2000.
15. D’Amico TA, Massey M, Herndon JE, et al: A biologic risk model for stage I lung cancer: Immunohistochemical analysis of 408 patients with the use of ten molecular markers. J Thorac Cardiovasc Surg 117:736-743, 1999.
16. Scher HI, Sarkis A, Reuter V, et al: Changing pattern of expression of the epidermal growth factor receptorand transforming growth factor alpha in the progression of prostatic neoplasms. Clin Cancer Res 1:545-550, 1995.
17. Gorgoulis V, Aninos D, Mikou P, et al: Expression of EGF, TGF-alpha and EGFR in squamous cell lung carcinomas. Anticancer Res 12:1183-1187, 1992.
18. Janas M, Bailey LR, Schmidt K, et al: Evaluation of epidermal growth factor receptor (EGFR) as a prognostic factor for survival in non-small cell lung Cancer (NSCLC) patients treated with platinum based chemotherapy as first line treatment (abstract 7024). Proc Am Soc Clin Oncol 23:619, 2004.
19. Arteaga CL: ErbB-targeted therapeutic approaches in human cancer. Exp Cell Res 284:122-130, 2003.
20. Carpenter G, Cohen S: Epidermal growth factor. Annu Rev Biochem 48:193-216, 1979.
21. O’Keefe E, Hollenberg MD, Cuatrecasas P: Epidermal growth factor, characteristics of specific binding in membranes from liver, placenta, and other target tissues. Arch Biochem Biophys 164:518-526, 1974.
22. Carpenter G: Epidermal growth factor: Biology and mechanism of action. Birth Defects Orig Artic Ser 16:61-72, 1980.
23. Soltoff SP, Cantley LC: p120cbl is a cytosolic adapter protein that associates with phosphoinositide 3-kinase in response to epidermal growth factor in PC12 and other cells. J Biol Chem 271:563-567, 1996.
24. Carpenter G: Employment of the epidermal growth factor receptor in growth factor independent signaling pathways. J Cell Biol 146:697-702, 1999.
25. Woodburn JR: The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther 82:241-250, 1999.
26. Nelson JM, Fry DW: Cytoskeletal and morphological changes associated with the specific suppression of the epidermal growth factor receptor tyrosine kinase activity in A431 human epidermoid carcinoma. Exp Cell Res 233:383-390, 1997.
27. Arteaga CL, Ramsey TT, Shawer LK, et al: Unliganded epidermal growth factor receptor dimerization induced by direct interaction of quinazolines with the ATP binding site. J Biol Chem 272:23247-23254, 1997.
28. Mendelsohn J, Baselga J: The EGF receptor family as targets for cancer therapy. Oncogene 19:6550-6565, 2000.
29. Wu X, Fan Z, Masui H, et al: Apoptosis induced by anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell lineand its delay by insulin. J Clin Invest 95:1897-1905, 1995.
30. Perrotte P, Matsumoto T, Inoue K, et al: Anti-epidermal growth factor receptor antibdy C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res 5:257-265, 1999.
31. Ciardiello F, Caputo R, Bianco R, et al: Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor tyrosine kinase inhibitor. Clin Cancer Res 7:1459-1465, 2001.
32. Mandal M, Adam L, Wang R-A: Inhibition of p-21 activated kinase 1, directional cell motility and invasion of growth factor activated human cancer cells by selective epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) ZD1839 (‘Iressa’) (abstract 786). Proc Am Assoc Cancer Res 43:157, 2002.
33. Baselga J, Norton L, Massui H: Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst 85:1327-1333, 1993.
34. Carpenter M, Buchsbaum DJ: Statistical growth curve modeling of tumors treated with Erbitux (IMC-C225) anti EGFR antibody, gemcitabine, and radiation (abstract 2386). Proc Am Assoc Cancer Res 43:1005-1006, 2002.
35. Cardiello F, Bianco R, Damiano V, et al: Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res 5:909-916, 1999.
36. Cardiello F, Caputo R, Bianco R, et al: Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res 6:2053-2063, 2000.
37. Raben D, Helfrich BA, Chan D, et al: ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, alone and in combinationwith radiation and chemotherapy as a new therapeutic strategy in non-small cell lung cancer. Semin Oncol 29(suppl 4):37-46, 2002.
38. Pollack VA, Savage DM, Baker DA, et al: Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: Dynamics of receptor inhibition in situ and anti-tumor effects in athymic mice. J Pharmacol Exp Ther 291:739-748, 1999.
39. Slichenmyer WJ, Elliott WL, Fry DW: CI-1033, a pan-Erb-B tyrosine kinase inhibitor. Semin Oncol 28:80-85, 2001.
40. Xia W, Mullin RJ, Keith BR, et al: Anti-tumor activity of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21:6255-6263, 2002.
41. Busam KJ, Capodieci P, Motzer R, et al: Cutaneous side effects in cancer patients treated with anti-epidermal growth factor receptor antibody C225. Br J Dermatol 144:1169-1176, 2001.
42. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and Cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
43. Saltz LB, Meropol NJ, Loehrer PJ, et al: Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201-1208, 2004.
44. Badarinath S, Mitchell EP, Jennis A, et al: Cetuximab plus FOLFOX for colorectal cancer (EXPLORE): Preliminary safety analysis of a randomized phase III trial. J Clin Oncol 22:3531, 2004.
45. Venook A et al: Phase III randomized study of fluorouracil and leucovorin calcium with irinotecan or oxaliplatin and with or without cetuximab in patients with previously untreated metastatic adenocarcinoma of the colon or rectum. Cancer and Leukemia Group B Protocol 80203.
46. Alberts S, Bernath A, et al: Phase III randomized study of irinotecan (CPT-11) and/or oxaliplatin (OXAL) plus 5-fluorouracil (5-FU)/leucovorin (CF) with or without cetuximab (C225) after curative resection for patients with stage III colon cancer. North Central Cancer Treatment Group Protocol N0147.
47. Tewes M, Schleucher N, Dirrsch O, et al: Results of a phase I trial of the humanized anti-epidermal growth factor receptor (EGFR) monoclonal antibody EMD 72000 in patients with EGFR-expressing solid tumors (abstract 378). Proc Am Soc Clin Oncol 21:95a, 2002.
48. Shin DM, Donato NJ, Perez-Soler R, et al: Epidermal growth factor receptor-targeted with C225 and cisplatin in patients with head and neck cancer. Clin Cancer Res 7:1204-1213, 2001.
49. Bruns CJ, Harbison MT, Davis DW, et al: Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res 6:1936-1948, 2000.
50. Prewett M, Rockwell P, Rockwell RF, et al: The biologic effects of C225, a chimeric monoclonal antibody to EGFR, on human prostate carcinoma. J Immunother Emphasis Tumor Immunol 19:419-427, 1996.
51. Robert F, Ezekiel MP, Spencer SA, et al: Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol 19:3234-3243, 2001.
52. Kies M, Arquette M, Nabell L, et al: Final report of the efficacy and safety of the anti-epidermal growth factor antibody Erbitux (IMC-C225), in combination with cisplatin patients with recurrent squamous cell carcinoma of the head and neck (SCCHN) refractory to cisplatin containing chemotherapy (abstract 925). Proc Am Soc Clin Oncol 21:232a, 2002.
53. Baselga J, Trigo J, Bourhis J, et al: Cetuximab (C225) plus cisplatin/carboplatin is active in patients (pts) with recurrent/metastaticsquamous cell carcinoma of the head and neck (SCCHN) progressing on the same dose and schedule platinum-based regimen (abstract 900). Proc Am Soc Clin Oncol 21:226a, 2002.
54 Trigo J, Hitt R, Koralewski P, et al: Ceutximab monotherapy is active in patients (pts) with platinum refractory recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN): Results of a phase II study (abstract 5502). Proc Am Soc Clin Oncol 223:487, 2004.
55. Rhoades CA, Kraut E, Schuller D, et al: Phase I and II study of OSI-774 and docetaxel in squamous cell carcinoma of the head and neck (SCCHN) (abstract 5541). Proc Am Soc Clin Oncol 23:496, 2004.
56. Burtness BA, Li Y, Flood W, et al: Phase III trial comparing cisplatin (C) + placebo (P) to C + anti-epidermal growth factor antibody (EGF-R) C225 in patients (pts) with metastatic recurrent head and neck cancer (HNC) (abstract 901). Proc Am Soc Clin Oncol 21:226a, 2002.
57. Soulieres D, Senzer NN, Vokes EE, et al: Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 19:3267-3279, 2004.
58. Bonner JA, Giralt J, Harari PM, et al: Cetuximab prolongs survival in patients with loco-regionally advanced squamous cell carcinoma of the head and neck: A phase III study of high dose radiation therapy with or without cetuximab (abstract 5507). J Clin Oncol 22(14S):489s, 2004.
59. Kane MA, Cohen E, List M, et al: Phase II study of 250 mg gefitinib in advanced squamous cell carcinoma of the head and neck (abstract 5586). Proc Am Soc Clin Oncol 23:507, 2004.
60. Cohen EEW, Stenson K, Gustin DM, et al: A phase II study of 250-mg gefitinib (ZD1839) monotherapy in recurrent or metastatic squamous cell carcinomaof the head and neck (SCCHN) (abstract 2021). Proc Am Soc Clin Oncol 22:502, 2003.
61. Humblet Y, Vega-Villegas E, Mesia R, et al: Phase I study of cetuximab in combination with cisplatin or carboplatinand 5-fluorouracil (5-FU) in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) (abstract 5513). J Clin Oncol 22(suppl):491s, 2004.
62. Robert F, Ezekiel MP, Spencer SA, et al: Phase I study of anti-epidermal growth factor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol 19:3234-3243, 2001.
63. Crombet T, Osorio M, Cruz T, et al: Use of the anti-EGFR antibody h-R3 in combination with radiotherapy in the treatment of advanced head and neck cancer (abstract 53). Proc Am Soc Clin Oncol 21:14a, 2002.
64. Cohen EE, Rosen F, Stadler WM, et al: Phase II trial of ZD1839 monotherapy in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 21:1980-1987, 2003.
65. Raben D, Kane M, Song J, et al: Preliminary report on toxicity of a phase I trial of gefitinib (Iressa) in combination with radiation/chemotherapy for patients with locally advanced head and neck cancer (LAHNC). Presented at an AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, Boston, November 17-21, 2003.
66. Senzer N, Soulieres D, Siu L, et al: Phase II evaluation of OSI-774, a potent oral antagonist of EGFR-TK in patients with advanced squamous cell carcinoma of the head and neck (abstract 6). Proc Am Soc Clin Oncol 20:2a, 2001.
67. Lillian L, Siu XC, Tsao M, et al: A phase I/II study of erlotinib (Tarceva) in combination with cisplatin in patients with recurrent or metastatic squamous cell cancerof the head and neck (HNSCC). Presented at an AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, Boston, November 17-21, 2003.
68. Herbst RS, Langer CJ: Epidermal growth factor receptors as a target for cancer treatment: The emerging role of IMC-C225 in the treatment of lung and head and neck cancers. Semin Oncol 29:27-36, 2002.
69. Rosell R, Daniel C, Ramlau R, et al: Randomized phase II study of cetuximab in combination with cisplatin (C) and vinorelbine (V) vs CV alone in first-line treatment of patients with epidermal growth factor receptor (EGFR)-expressing advanced non-small cell lung cancer (NSCLC) (abstract 7012). Proc Am Soc Clin Oncol 23:618, 2004.
70. Kim ES, Mauer AM, Tran HT, et al: A phase II study of cetuximab, an epidermal growth factor receptor (EGFR) blocking antibody, in combination with docetaxel in chemotherapy refractory/resistant patients with advanced non-small cell lung cancer: Final report (abstract 2581). Proc Am Soc Clin Oncol 22:642, 2003.
71. Fukuoka M, Yano S, Giaccone G, et al: Multiinstitutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small cell lung cancer. J Clin Oncol 21:2237-2246, 2003.
72. Giaccone G, Herbst RS, Manegold C, et al: Gefitinib in combination with gemcitabine and cisplatin in advanced non-small cell lung cancer: A phase III trial-INTACT1. J Clin Oncol 22:777-784, 2004.
73. Kris MG, Natale RB, Herbst RS, et al: Efficacy of gefitinib, an inhibitor of epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA 290:2149-2158, 2003.
74. Perez-Soler R, Chachoua A, Huberman M, et al: Final results of a phase II study of erlotinib (Tarceva) monotherapy in patients with advanced non-small cell lung cancer following failure of a platinum-based chemotherapy (abstract P-611). Lung Cancer 41(suppl 2):S246, 2003.
75. Shepherd FA, Pereira J, Giulianu TE, et al: A randomized placebo-controlled trial of erlotinib in patients with advanced non-small cell lung cancer (NSCLC) following failure of first line or second line chemotheapy. A national Cancer Institute of Canada clinical trials group (NCIC CTG) trial. Presented at the 40th Annual Meeting of the American Society of Clinical Oncology, New Orleans, June 5-8, 2004.
76. Herbst RS, Giaccone G, Schiller JH, et al: Gefitinib in combination with paclitaxel and carboplatin in advanced non-small cell lung cancer: A phase III trial-INTACT 2. J Clin Oncol 22:785-794, 2004.
77. Gatzemeier U, Pluzanska A, Szczesna A, et al: Results of a phase III trial of erlotinib (OSI-774) combined with cisplatin and gemcitabine (GC) chemotherapy in advanced non-small cell lung cancer (NSCLC) (abstract 7010). Proc Am Soc Clin Oncol 23:617, 2004.
78. Herbst RS, Prager D, Hermann R, et al: TRIBUTE-a phase III trial of erlotinib HCl (OSI-774) combined with carboplatin and paclitaxel (CP) chemotherapy in advanced non-small cell lung cancer (NSCLC) (abstract 7011). Proc Am Soc Clin Oncol 23:617, 2004.
79. Hidalgo M, Siu LL, Nemunaitis J, et al: Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 19:3267-3279, 2001.
80. Herbst RS, Prager D, Hermann R, et al: TRIBUTE: A phase III trial of erlotinib HCl (OSI-774) combined with carboplatin and paclitaxel (CP) chemotherapy in advanced non-small cell lung cancer (NSCLC) (abstract 7011). Proc Am Soc Clin Oncol 23:617, 2004.
81. Janas M, Bailey LR, Schmidt K, et al: Evaluation of epidermal growth factor receptor (EGFR) as a prognostic factor for survival in non-small cell lung cancer (NSCLC) patients treated with platinum-based chemotherapy as first-line treatment (abstract 7024). Proc Am Soc Clin Oncol 23:619, 2004.
82. Shin DM, Nemunaitis J, Zinner RG, et al: A phase I clinical and biomarker study of CI-1033, a novel pan-erb-B tyrosine kinase inhibitor in patients with solid tumors (abstract 324). Proc Am Soc Clin Oncol 20:82a, 2001.
83. Simon GR, Olson S, Garrett CR, et al: A phase I pharmacokinetic (PK) and safety study of intravenous (IV) CI-1033 in patients with advanced solid tumors (abstract 3057). Proc Am Soc Clin Oncol 23:209, 2004.
84. Wakeling AE, Guy SP, Woodburn JR, et al: ZD1839 (Iressa): An orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res 62:5749-5754, 2002.
85. Lynch TJ, Bell DW, Sordella R, et al: Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small cell lung cancer to gefitinib. N Engl J Med 350:2129-2139, 2004.
86. Paez JG, Janne PA, Lee JC, et al: EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 304:1497-1500, 2004.
87. Pao W, Zakowski M, Cordon-Cardo C, et al: Molecular characteristics of non-small cell lung cancer (NSCLC) patients sensitive to gefitinib (abstract 7025). Proc Am Soc Clin Oncol 23:619, 2004.
88. Kim YH, Ishii G, Goto K, et al: Dominant papillary subtype is a significant predictor of the response to gefitinib in adenocarcinoma of the lung. Clin Cancer Res 10:7311-7317, 2004.
89. Miyoshio T, Satoh Y, Okumura S, et al: Early stage lung adenocarcinoma with a micropapillary pattern, a distinct pathologic marker for a significant poor prognosis. Am J Surg Pathol 27:101-109, 2003.
90. Janne PA: EGFR mutations predict response to gefitinib-now what? Presented at the 40th Annual Meeting of the American Society of Clinical Oncology, New Orleans, June 5-8, 2004.
91. Clark G, Perez-Soler R, Siu L, et al: Rash severity is predictive of increased survival with erlotinib HCl (abstract 786). Proc Am Soc Clin Oncol 22:196, 2003.