RIBBON-1 results bolster bevacizumab as add-on to standard Rx for metastatic breast cancer
ORLANDO-RIBBON-1 results demonstrate that bevacizumab (Avastin) can be added to nearly any standard chemotherapy regimen for metastatic breast cancer, although the jury is still out on how meaningful the additional treatment is for overall survival.
ORLANDO-RIBBON-1 results demonstrate that bevacizumab (Avastin) can be added to nearly any standard chemotherapy regimen for metastatic breast cancer, although the jury is still out on how meaningful the additional treatment is for overall survival.

The international trial was lead by Nicholas J. Robert, MD, of Fairfax Northern Virginia Hematology Oncology. For the study, 1,237 patients in 22 countries were randomized to receive either bevacizumab or placebo plus chemotherapy. Prior to randomization, investigators chose capecitabine (Xeloda), a taxane, or an anthracycline-based chemotherapy regimen (see Table 1).
Eligibility criteria included metastatic breast cancer or locally recurrent disease, no prior cytotoxic treatment, and HER2-negative disease. At progression, all patients were eligible to receive bevacizumab as second-line therapy. The median follow-up time was 15.6 months in the capecitabine cohort and 19.2 months in the pooled taxane or anthracycline cohort (ASCO 2009 abstract 1005).
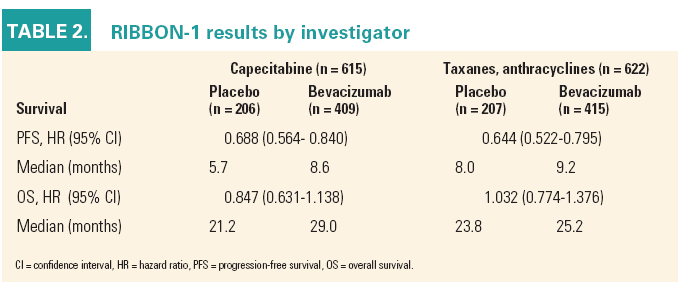
According to the results, the addition of bevacizumab resulted in statistically significant improvement in progression-free survival (PFS), with a 45% improvement in PFS in the capecitabine plus bevacizumab arm and a 55% improvement in the bevacizumab plus taxane or anthracycline arm, said Philippe Bishop, MD, vicepresident, clinical development, Avastin at Genentech (See Table 2).

“It’s the third positive study that we have in Avastin in advanced, HER2-negative breast cancer,” Dr. Bishop said. “For patients, it’s another piece of information that when Avastin is added to a standard of care regimen for metastatic breast cancer, there is a measurable clinical benefit.”
Both the E2100 and AVADO trials added bevacizumab, at different doses, to a taxane (paclitaxel in E2100; docetaxel [Taxotere] plus placebo in AVADO). Both studies showed a significant improvement in PFS (N Engl J Med 357:2666-2676, 2007; ASCO 2008 LBA1011).
The E2100 study formed the basis for the approval of bevacizumab in the treatment of metastatic breast cancer in Europe and the U.S., although approval in the latter was accelerated rather than full. Results from AVADO and RIBBON-1 will be used to request full FDA approval.
Commentary
During an ASCO highlights session, Clifford Hudis, MD, offered his take on the RIBBON-1 results. “This trial...reflected real-world practice because the investigators were able to select anthracyclines or taxanes or capecitabine. The results provide additional evidence of a modest, but consistent, effect for bevacizumab in the first-line setting with almost any of our standard chemotherapy drugs,” he said.

However, as with the results from AVADO and E2100, adding bevacizumab did not significantly affect overall survival, Dr. Hudis pointed out, which speaks to the debate in the oncology community “over our inability to change survival in first-line treatment of metastatic breast cancer.” Dr. Hudis is chief of the Breast Cancer Medicine Service at New York’s Memorial Sloan-Kettering Cancer Center.
The lack of meaningful data on overall survival has been a consistent criticism of studies pairing bevacizumab with standard therapy. Previous studies demonstrated that the combination of bevacizumab with paclitaxel, as well as bevacizumab with capecitabine, improved PFS but did not increase overall survival.
Dr. Bishop addressed the issue of overall survival. “These trials were designed with PFS as the primary endpoint. If we tried to design this trial for overall survival, we would have to include thousands of patients,” he said. “PFS is a direct measure of what the treatment is doing. The way to look at survival is to make sure that there is not a detriment to the patient with this treatment. The results are telling us that, in terms of overall survival, we’re not harming patients.”