Treatment varies widely in chronic myeloid leukemia
ORLANDO-The therapeutic pathways used for chronic myeloid leukemia patients with suboptimal response to 400 mg/d imatinib (Gleevec) tend to vary, despite the existence of NCCN clinical practice guidelines.
ABSTRACT: NCCN guidelines that call for switching and dose escalation may go unheeded by community oncologists
ORLANDO-The therapeutic pathways used for chronic myeloid leukemia patients with suboptimal response to 400 mg/d imatinib (Gleevec) tend to vary, despite the existence of NCCN clinical practice guidelines.
According to a duo of ASCO 2009 presentations, some patients are not being dose escalated on imatinib prior to switching to dasatinib (Sprycel). Also, switching to dasatinib was found to be associated with more adverse events than dose escalation of imatinib.
“These studies give us a window on real-world clinical practice and suggest that we need to make greater efforts to educate patients and providers on how to follow NCCN guidelines,” said the senior author of both posters, James Griffin, MD, director of medical oncology at Dana-Farber Cancer Center and professor of medicine at Harvard Medical School, both in Boston.
The first study retrospectively analyzed two large administrative health claims databases and showed that most patients remained on their inital imatinib dose. Among the patients who switched to dasatinib, some did not increase the dose of imatinib before switching and a sizeable proportion had to switch back to imatinib (abstract 7090). The study took place from June 2002 to July 2008 and included 5,159 CML patients who initiated treatment on imatinib. Of these, 1,054 either had dose escalation of imatinib (n = 839) or were switched to dasatinib (n = 305). Among all 5,159 CML patients initiated on imatinib, 12.1% discontinued by the end of the first year, and 25.4% by the end of the second year.

“We need to better understand the factors behind this discontinuation pattern and educate patients to be compliant with their CML treatment,” said study coauthor Vamsi Bollu PhD, MBA, associate director, health economics and outcomes research for Novartis’ Oncology U.S. Clinial Development and Medical Affairs.
Among the 305 switchers, 115 (37.7%) had an imatinib dose increase prior to the switch and 66 (21.6%) were escalated to 800 mg of imatinib prior to the switch. Fifty-one patients (16.7%) had used imatinib for less than six months before switching, and 17.1% switched back to imatinib within six months of dasatinib treatment.
In the second study, on adverse events, patients who switched to dasatinib experienced significantly more adverse events compared with patients who dose escalated on imatinib. The analysis was based on the same administrative claims databases as the first study (abstract 7092).

“It is not surprising that switchers had more side effects than dose escalators. Usually side effects are more frequent with dasatanib, and many patients switch back to imatinib,” Dr. Griffin said. “It’s important to be aware of this, because patients have the option to dose escalate or switch to another drug. Oncologists need to know that if you escalate imatinib, you are likely to have fewer side effects than if you switch to dasatinib. You can always switch the patient after dose escalation if response to higher dose imatinib is suboptimal.”
The study had two cohorts of patients: switchers (n = 175), who did not escalate imatinib before switching to dasatinib but could have escalated to a dose below 800 mg prior to switching, and escalators (n = 474), who increased the initial dose of imatinib by at least 100 mg imatinib and did not switch to imatinib during the study period. Based on medical and pharmacy claims, patients were monitored for common adverse events for imatinib and dasatinib such as fluid retention, cytopenias, and nonhematologic adverse events. The latter included gastrointestinal events, headache, fatigue, dyspnea, pruritus, and myalgia.
During the follow-up period after the index date, switchers had a significantly higher risk of developing one or more of the studied adverse events compared with escalators. Significantly more fluid retention, thrombocytopenia, neutropenia, dyspnea, constipation, nausea and vomiting, and congestive heart failure were observed in switchers than in escalators.
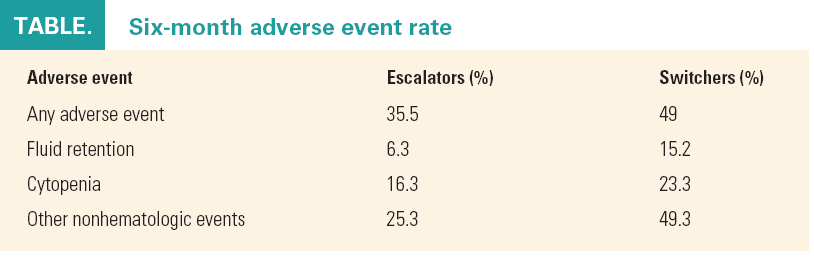
The authors acknowledged that these data do not provide information on whether prescribed drugs are actually taken according to instructions. Also, all of the adverse events that were studied might not have been captured by claims data. Finally, the study did not include nilotinib switchers because there were too few of them.