Rituximab Shows Promise in Treating ITP
ORLANDO, Florida-Rituximab (Rituxan) shows promise in the treatment of immune thrombocytopenic purpura (ITP), according to two poster presentations at the 43rd Annual Meeting of the American Society of Hematology (ASH). Nichola Cooper, MRCP, fellow, and James B. Bussel, MD, professor of pediatrics, both at Weill Medical College of Cornell University, and Mansoor N. Saleh, MD, professor of medicine at the University of Alabama at Birmingham, reported the results of separate studies investigating the efficacy and toxicity of rituximab in adults with refractory ITP. Rituximab binds to the antigen CD20 and depletes circulating B-lymphocyte cells. "In theory," Dr. Cooper said, "if you get rid of the B cells, it decreases the autoimmune response."
ORLANDO, FloridaRituximab (Rituxan) shows promise in the treatment of immune thrombocytopenic purpura (ITP), according to two poster presentations at the 43rd Annual Meeting of the American Society of Hematology (ASH). Nichola Cooper, MRCP, fellow, and James B. Bussel, MD, professor of pediatrics, both at Weill Medical College of Cornell University, and Mansoor N. Saleh, MD, professor of medicine at the University of Alabama at Birmingham, reported the results of separate studies investigating the efficacy and toxicity of rituximab in adults with refractory ITP. Rituximab binds to the antigen CD20 and depletes circulating B-lymphocyte cells. "In theory," Dr. Cooper said, "if you get rid of the B cells, it decreases the autoimmune response."
Cornell Study
The Cornell team administered rituximab at 375 mg/m² once a week for 4 weeks to 23 patients who were refractory to at least one prior ITP treatment, had a platelet count of less than 30,000/µL, and were not infected with the human immunodeficiency virus (HIV). Twenty-one patients were evaluable after more than 9 weeks since the first infusion.
Ten patients (47%) had a good response to the drug, obtaining platelet levels of more than 50,000/µL on two counts, 1 week apart. In eight of these patients (38% of the total study population), ITP completely resolved, with platelet levels of more than 150,000/µL on both counts.
Patients responded at different rates, some earlier in treatment and others later. One person who responded to the drug relapsed 48 weeks after her first infusion and was successfully retreated. The trial demonstrated rituximab’s effectiveness in adults who have failed steroid treatment but have not undergone splenectomy.
"The most important finding is the long duration of activity and the low toxicity to patients," Dr. Cooper said. "Some patients go longer than a year without [additional] treatment and maintain counts above 50,000/µL. Most are up to normal levels."
Patients in both studies experienced infusion reactions, including red face, hives, pruritus, and throat discomfort, but no other side effects were noted. "The fact that there was no infection and no decrease in immunoglobulins is surprising," Dr. Cooper said. "We’re always concerned about long-term consequences but don’t know what they may be."
University of Alabama Study
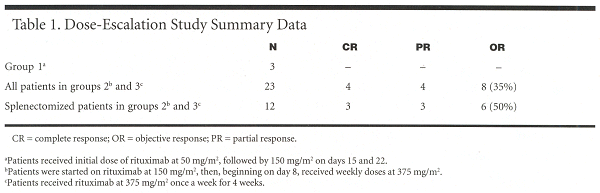
In Dr. Saleh’s dose-escalation study, three patients received an initial dose of rituximab 50 mg/m², followed by 150 mg/m² on days 15 and 22. Another three patients were started on rituximab at 150 mg/m², then, beginning on day 8, were given weekly doses of rituximab at 375 mg/m². Twenty patients received the standard dose of 375 mg/m² once a week for 4 weeks.
Eligible patients included ITP patients with platelet counts of less than 75,000/µL, who had failed steroid treatment or were unable to maintain a platelet count greater than 75,000/µL at tolerable doses, had bone marrow with elevated megakaryocytes, and had standard hemoglobin and white blood cell counts. Most patients responded within 2 to 4 weeks of the start of treatment.
"We clearly saw better results with patients who had been splenectomized, even in this limited sample size. Responses were seen in patients who received the 150-mg/m² dose on week 1 followed by three weekly doses of 375 mg/m², as well as the 375 mg/m² every week × 4 full-dose treatment," Dr. Saleh said.
‘‘Half the splenectomized patients (6 of 12) responded to treatment.’’ (See Table 1.)
Rituximab could have far-reaching benefits, and according to Dr. Saleh, "It could work on other diseases where B cells are important, such as lupus, rheumatoid arthritis, or other autoimmune conditions."