The Role of Bisphosphonates in Early- and Advanced-Stage Breast Cancer: Have We Finally Optimized Care?
Bisphosphonates have played an important role in the treatment of breast cancer, mainly in patients with bone metastasis, by reducing the risk of fracture, spinal cord compression, and hypercalcemia.
Table 1: Comparison of Commonly Used Bisphosphonates vs Placebo at Recommended Dosing, and Overall Risk of Skeletal-Related Events in Patients With Metastatic Breast Cancer and Bone Metastasis
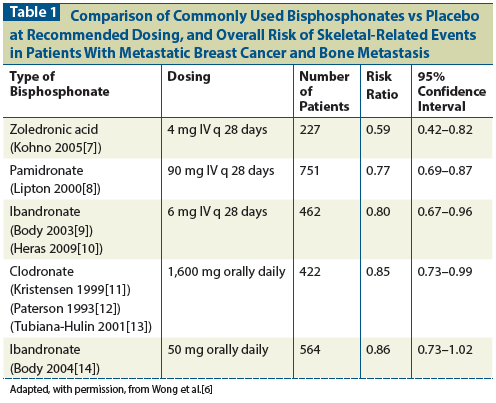
Table 2: Canadian Pricing of Commonly Used Bisphosphonates and Denosumab for the Treatment of Metastatic Breast Cancer Based on Current Standard Dosing Guidelines, Including Cumulative Drug, Pharmacy, and Administration Costs
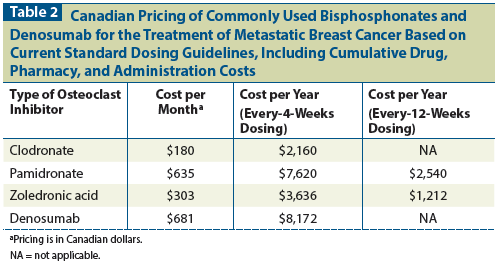
Table 3: Major Adjuvant Studies of Oral Clodronate for Early-Stage Breast Cancer
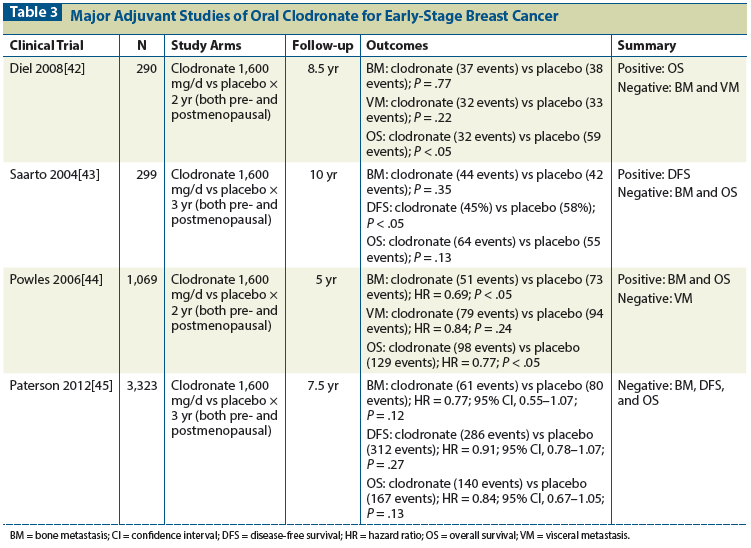
Table 4: Major Adjuvant Studies of Zoledronic Acid for Early-Stage Breast Cancer
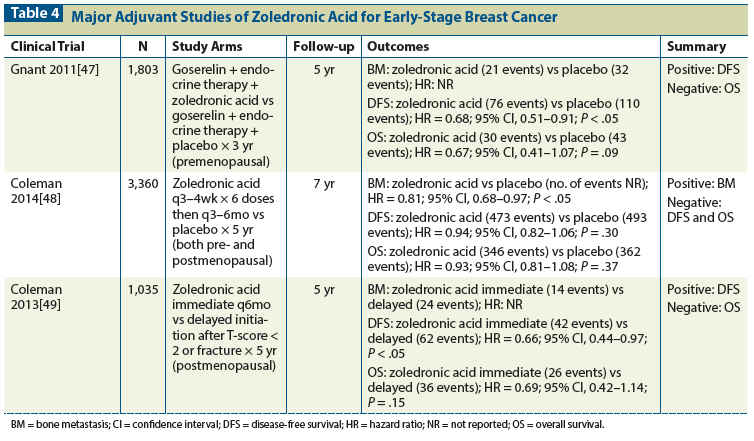
Table 5: Early Breast Cancer Trialists’ Collaborative Group (EBCTCG ) Meta-Analysis on Effects of Bisphosphonate Treatment on Recurrence and Cause-Specific Mortality in Early Breast Cancer by Menopausal Status
Bisphosphonates have played an important role in the treatment of breast cancer, mainly in patients with bone metastasis, by reducing the risk of fracture, spinal cord compression, and hypercalcemia. Both oral and intravenous products are available and have strong supporting clinical evidence. Zoledronic acid, the most frequently used intravenous agent, has traditionally been given on a monthly dosing schedule. A novel every-12-weeks maintenance dosing schedule shows clinical equivalence and promises to improve safety, affordability, and quality of care. The Early Breast Cancer Trialists’ Collaborative Group has recently helped clarify the controversial benefit of bisphosphonates in the adjuvant setting. Based on their meta-analysis, we now have strong evidence supporting the use of adjuvant bisphosphonates to help prevent the development of bone metastasis in postmenopausal breast cancer patients, which leads to a significant improvement in breast cancer–specific survival. We eagerly await the full publication of this practice-changing study, which continues the incremental advance of the treatment of patients with breast cancer.
Introduction
Bone is the primary site for breast cancer metastasis, representing a significant burden of disease and leading to fractures, spinal cord compression, pain, and hypercalcemia.[1] Breast cancer patients often live many years with skeletal metastasis, with an average survival of 4 years.[2] The propensity of breast cancer to metastasize to bone likely represents a complex interaction between metastatic cancer cells, bone, and the intermediary local microenvironment. Bone-targeted therapy with bisphosphonates has greatly improved the systemic management of patients with advanced breast cancer by decreasing the frequency and morbidity of skeletal-related events (SREs). We also now have emerging evidence that demonstrates the benefit of adjuvant bisphosphonates in preventing bone metastasis and improving breast cancer–specific survival among postmenopausal women who undergo adjuvant therapy.
Mechanism of Action
Bisphosphonates are structural analogs of pyrophosphate that embed into bone, binding to hydroxyapatite and inhibiting osteoclast activity and survival.[3,4] Thus, bisphosphonates can inhibit tumor-mediated osteolysis driven by osteoclast activation. There are two classes of bisphosphonates-(1) nitrogen-containing and (2) non–nitrogen-containing-which differ in potency and direct mechanisms of action. Bisphosphonates have also been shown to have effects independent of their antiresorptive properties, including evidence of immune-modulatory properties. Bisphosphonates may also have associated antiproliferative and antiangiogenic effects.[5] These multiple mechanisms of action speak to the importance of bisphosphonates as bone-modifying agents and likely contribute to their significant clinical benefit in the treatment of breast cancer.
Role of Bisphosphonates in Advanced-Stage Breast Cancer
Efficacy
Strong evidence supports the role of bisphosphonates in the treatment of advanced breast cancer. A Cochrane Collaboration systematic review and meta-analysis of 9 studies, which included 2,806 patients, demonstrated that bisphosphonates decreased the SRE rate by 15% compared with placebo in women with breast cancer who had bone metastasis (relative risk [RR] = 0.85; 95% confidence interval [CI], 0.77–0.94).[6] All bisphosphonates were effective (clodronate, pamidronate, ibandronate, and zoledronic acid) and reduced SREs by 20% to 40%, depending on the agent (Table 1).[7-14] Because of the differing study populations, we should be cautious in drawing conclusions regarding the superiority of one agent over another; however, zoledronic acid had the lowest risk ratio, 0.59 (95% CI, 0.42–0.82). No significant difference was seen between oral agents (RR = 0.84; 95% CI, 0.76–0.93) and intravenous agents (RR = 0.83; 95% CI, 0.72–0.95). Only one clinical trial involving zoledronic acid was included in this subanalysis, however.[7] The Cochrane Collaboration meta-analysis does not show an overall survival benefit for the use of bisphosphonates in women with breast cancer and bone metastasis (RR = 1.01; 95% CI, 0.92–1.11). In addition, the review does not show consistent improvement in global quality of life or improvement in bone pain associated with bisphosphonate therapy.
A number of studies have compared specific bisphosphonates directly against one another. There are no large prospective controlled trials comparing the effectiveness of oral clodronate with that of pamidronate. One small study suggests the potential superiority of pamidronate over clodronate in reducing the risk of vertebral fractures and improving self-reported pain scores.[15] However, the results of this study were never fully published. Pamidronate has also shown superiority over intravenous clodronate in the treatment of malignant hypercalcemia.[16] A large study compared the long-term safety and efficacy of zoledronic acid and pamidronate.[17] The number of SREs in the zoledronic acid and pamidronate groups were similar: 46% and 49%, respectively. The skeletal morbidity rate was lower in patients receiving zoledronic acid (0.90 events per year, compared with 1.49), but this did not reach statistical significance (P = .125). However, a multiple-event analysis showed a significant 20% decrease in SREs with zoledronic acid as compared with pamidronate. The safety profiles of both agents were similar. The recent Zoledronate Versus Ibandronate Comparative Evaluation (ZICE) trial compared oral ibandronate and zoledronic acid and did not conclude noninferiority.[18] The annual SRE rate was 0.50 with ibandronate (95% CI, 0.45–0.55) and 0.44 with zoledronic acid (95% CI, 0.39–0.48). Less renal toxicity was seen in the ibandronate arm.
In a large randomized controlled trial that included more than 1,000 patients, the effectiveness of zoledronic acid was compared with that of denosumab, a monoclonal immunoglobulin G2 antibody that inhibits activation of the receptor activator of nuclear factor kappa-B ligand (RANKL). This study showed the superiority of denosumab in delaying the time to first SRE (hazard ratio [HR] = 0.82; 95% CI, 0.71–0.95; P = .01) and time to subsequent SREs (rate ratio = 0.77; 95% CI, 0.66–0.89; P = .001).[19] However, overall survival, disease progression, and rate of adverse events were similar between the groups. Only a very modest improvement in health-related quality of life was noted, favoring the use of denosumab.[20]
The National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology (ASCO), and the European Society of Medical Oncology (ESMO) are consistent in recommending either zoledronic acid or denosumab.[21-23] The recommended dosage of zoledronic acid is 4 mg every 3 to 4 weeks. The current NCCN task force report and ASCO guidelines also list pamidronate as an alternative choice of therapy.[21,23]
Side effects and toxicity
The side-effect profiles of bisphosphonates and denosumab are clearly established, and both are generally well tolerated. Bisphosphonates may transiently cause an influenza-like reaction and fever following administration. A number of bisphosphonates are associated with a risk of renal toxicity and may lead to an elevation in serum creatinine in approximately 5% to 10% of patients, particularly those receiving zoledronic acid.[17,24]. The risk of renal toxicity appears to be related to dose, route of administration, and hydration and may be lower with oral bisphosphonates.[25] Clinical practice guidelines recommend lengthened infusion times and dose reductions in the setting of renal insufficiency with a creatinine clearance between 30 and 60 mL/min.[21] Treatment is not usually recommended in patients with a creatinine clearance ≤ 30 mL/min. The development of long-term renal dysfunction or failure, especially requiring dialysis, is rare.[26]
There is a small risk of osteonecrosis of the jaw with bisphosphonates and denosumab, which appears to increase with cumulative long-term therapy.[27,28] Atypical fractures, usually subtrochanteric and diaphyseal fractures of the femur, have also been reported with long-term bisphosphonate use.[29] Denosumab poses a risk of severe hypocalcemia, which may result in serious and potentially life-threatening cardiac arrhythmias.[30,31] Therefore, calcium and vitamin D supplementation is required, and denosumab is contraindicated in patients with severe renal impairment. Denosumab is also given by subcutaneous injection, which may be more advantageous than intravenous infusion.
Choosing wisely
Cost-effectiveness remains a major issue for bone-modifying agents, given their high cost and the need for long-term use. Bisphosphonates have demonstrated clear cost-effectiveness compared with best supportive care by decreasing the rate of skeletal complications and preventing the associated need for hospitalization, radiation, and surgery.[32] Multiple bisphosphonates and denosumab are now available to treat bone metastasis, which has led to much debate-particularly in government-funded or resource-limited healthcare systems-about which agents represent the best value. In Table 2, we have outlined approximate drug and administration costs for clodronate, pamidronate, zoledronic acid, and denosumab at our center (based on Canadian pricing). Analyses have shown that oral agents such as clodronate and ibandronate appear more cost-effective compared with intravenous bisphosphonates; however, oral agents are less potent than intravenous bisphosphonates and may have inferior efficacy.[33,34] Studies of the cost-effectiveness of zoledronic acid and denosumab have also shown conflicting results.[35-37] Overall, bisphosphonates have clearly documented cost-effectiveness despite their high direct individual drug costs. The incremental benefit of one agent over another is likely less important than establishing long-term compliance with therapy. Therefore, side effects, ease of administration, and affordability should guide patient and physician choices. Direct comparisons of specific agents are also difficult because drug prices will continue to change over time and also across countries, especially once generic drug formulations become available. There is also emerging evidence to support less frequent dosing schedules for intravenous bisphosphonates, which would greatly improve cost-effectiveness and affordability, allowing for more wide-scale use among breast cancer patients.
Interval dosing and duration of therapy
The frequency of administration of intravenous bisphosphonates has been questioned based on pharmacokinetics and long-term bone deposition. The ZOOM and OPTIMIZE-2 trials randomized patients with metastatic breast cancer to every-4-weeks or every-12-weeks dosing schedules of zoledronic acid after 1 year of standard bisphosphonate therapy.[38,39] The results of the ZOOM trial showed a skeletal morbidity rate of 0.22 (95% CI, 0.14–0.29) with the standard every-4-weeks dosing schedule vs 0.26 (95% CI, 0.15–0.37) with the every-12-weeks maintenance dosing schedule. The ZOOM study does show relative clinical equivalency; however, statistical noninferiority was not proved because of the small sample size.[38] The recent OPTIMIZE-2 trial did demonstrate noninferiority: The SRE rate was 22% with an every-4-weeks dosing schedule vs 23.2% with an every-12-weeks maintenance dosing schedule (difference: −1.2%; 95% CI, −7.5% to 9.8%; P = .724).[39] Patients in the every-12-weeks group had fewer renal adverse events and no reported instances of osteonecrosis of the jaw. This promising every-12-weeks maintenance dosing strategy will further improve the cost-effectiveness of bisphosphonates and reduce the risk of cumulative dose toxicity. Further study is needed to determine whether denosumab could be used in a similar maintenance dosing strategy.
In contrast, the BISMARK trial did not meet its noninferiority endpoint.[40] This was partially related to poor enrollment; however, the study authors also concluded that the marker-adapted dosing strategy may in fact be suboptimal. The choice of dosing strategy was based on measurement of urinary N-telopeptide of type 1 collagen (NTX), a commonly used biomarker of bone turnover and remodeling.
Clinical practice guidelines could adopt this every-12-weeks maintenance bisphosphonate dosing strategy once the ZOOM and OPTIMIZE-2 study results are fully published. A number of centers have already adopted this maintenance dosing schedule based on the preliminary results that show clinical equivalence. The large ongoing Cancer and Leukemia Group B (CALGB) 70604 trial is investigating every-4-weeks vs every-12-weeks dosing schedules of zoledronic acid in breast cancer patients with newly diagnosed bone metastasis.[41] This will help clarify whether every-12-weeks dosing schedules for intravenous bisphosphonates should become the standard of care for the treatment of bone metastasis.
The necessary duration of bisphosphonate therapy also remains unclear. For now, treatment with a maintenance strategy until there is a significant decline in the patient’s performance status would be reasonable. Most clinical trials have not used bisphosphonates for durations longer than 2 to 3 years. Long-term use of bisphosphonates in the setting of osteoporosis has raised concern about subtrochanteric and femoral shaft fractures.[29] Therefore, further study is needed to define the exact duration of therapy in the context of metastatic cancer, especially as overall survival with metastatic disease continues to improve.
Summary
Overall, treatment decisions should be individualized according to each patient’s clinical presentation, comorbidities, performance status, and optimal method of administration. Oral bisphosphonates such as clodronate and ibandronate are reasonable treatment alternatives for patients with limited bone disease who wish to avoid repeated hospital visits for injections or intravenous infusions. They are also good alternatives for resource-limited countries, where the higher costs of pamidronate, zoledronic acid, and denosumab may be prohibitive. For patients who present with more advanced disease or hypercalcemia or who prefer more aggressive management, we would recommend zoledronic acid or denosumab because of superior relative efficacy. We believe an every-12-weeks maintenance dosing schedule for zoledronic acid could be recommended after 1 year of therapy in patients with otherwise stable bone disease. We also look forward to future studies that may establish every-12-weeks dosing schedules as the standard of care for all patients with metastatic bone disease. This may make zoledronic acid a more attractive treatment option because of both patient convenience and cost to the healthcare system, compared with denosumab.
Role of Bisphosphonates in Early-Stage Breast Cancer
The use of bisphosphonates in early-stage breast cancer has been more controversial. A number of studies have proposed that bisphosphonates alter the local tissue microenvironment and exert anticancer effects that may delay or prevent the establishment of bone metastasis.
Bisphosphonates have been clearly shown to reduce the rate of bone loss associated with breast cancer treatment. However, studies have been conflicting regarding their role as an adjuvant anticancer therapy. Currently, clinical practice guidelines do not recommend the use of bisphosphonates in early-stage breast cancer either to prevent the formation of bone metastasis or to improve survival outcomes.[21-23] However, these guidelines need to be updated to reflect more recent advances in knowledge.
Clodronate
The earliest adjuvant trials in early-stage breast cancer patients used oral clodronate at a dosage of 1,600 mg/d for 2 to 3 years. Major trials are outlined in Table 3.[42-45] The original study by Diel et al[46] in 1998 showed great promise, with significant reductions in bone and visceral metastasis and improvement in overall survival. Of note, this study was conducted in a group of high-risk breast cancer patients with documented tumor cells within the bone marrow before the start of adjuvant therapy. Long-term follow-up at 8.5 years showed the overall survival benefit was maintained; however, the reduced incidence of bone and visceral metastasis was no longer observed.[42]
The results of subsequent studies using adjuvant clodronate have been inconsistent. The largest study to date investigating adjuvant oral clodronate in breast cancer is the National Surgical Adjuvant Breast and Bowel Project (NSABP) B34 trial, which enrolled more than 3,000 patients.[45] This trial was originally negative for all outcome measures, including bone metastasis–free interval, recurrence-free survival, and overall survival. However, subgroup analysis in postmenopausal women did show a 25% improvement in recurrence-free survival. This included reductions in the development of both bone and visceral metastases. No overall survival benefit was demonstrated in postmenopausal women, probably because of the low event rate observed in trial participants.
Zoledronic acid
More recent studies using zoledronic acid in the adjuvant treatment of breast cancer are summarized in Table 4.[47-49] These studies have also had mixed results but have provided more clarity on the role of bisphosphonates in the adjuvant setting. The similarity of the overall results to results of previously reported studies using oral clodronate suggests a benefit for adjuvant bisphosphonate therapy in postmenopausal women. The ZO-FAST trial showed a significant benefit for early vs delayed use of zoledronic acid in postmenopausal women, with improved disease-free survival (HR = 0.66; 95% CI, 0.44–0.97; P = .038). No overall survival benefit was observed (HR = 0.69; 95% CI, 0.42–1.14; P = .15).[49] The AZURE trial was negative for its primary and secondary survival outcomes, except for a significant reduction in the development of bone metastasis (HR = 0.81; 95% CI, 0.68–0.97; P = .02). However, a preplanned subgroup analysis did report a significant improvement in disease-free survival in postmenopausal women.[48] Therefore, the overall negative study results likely reflect the significant proportion of premenopausal women enrolled in the trial who did not derive any benefit from adjuvant zoledronic acid. The Austrian Breast and Colorectal Cancer Study Group Trial 12 (ABCSG-12), conducted in premenopausal breast cancer patients undergoing ovarian suppression, did report a significant improvement in disease-free survival (HR = 0.68; 95% CI, 0.51–0.91; P = .009).[47] This benefit may be attributed to the associated use of ovarian suppression, which created an artificial postmenopausal state in these young women. Overall, these three large trials were consistent in demonstrating a clinical benefit in postmenopausal women and in premenopausal women undergoing ovarian suppression.
Early Breast Cancer Trialists’ Collaborative Group
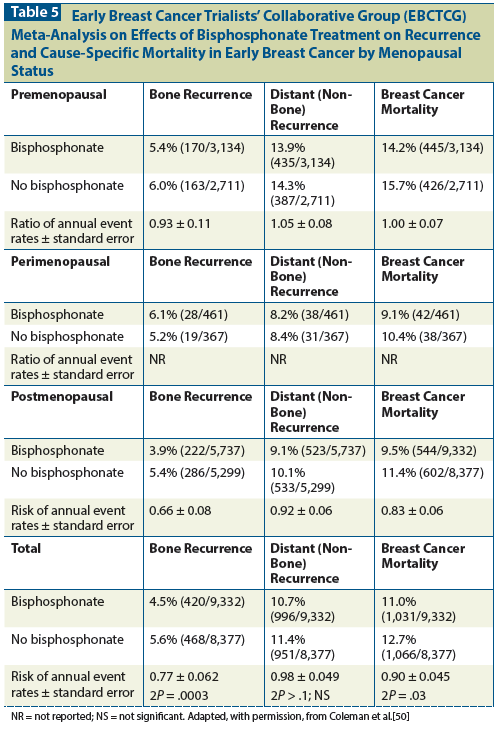
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis provides greater clarity about the role of adjuvant bisphosphonate therapy in breast cancer. The EBCTCG meta-analysis, which included 17,709 women treated with adjuvant bisphosphonates, indicated a significant improvement in the prevention of both distant disease recurrence (18.4% vs 21.9%; absolute net gain +3.5% [SE, 1.2]; log rank 2P = .0003) and bone metastasis (5.9% vs 8.8%; absolute net gain +2.9% [SE, 0.8]; log rank 2P < .0001) in postmenopausal women at 10 years.[50] The improvements in breast cancer mortality (15.2% vs 18.3%; absolute net gain +3.1% [SE, 1.3]; log rank 2P = .004) and all-cause mortality (21.5% vs 23.8%; absolute net gain +2.3% [SE, 1.5]; log rank 2P = .007) were also significant. In this analysis, women aged > 55 years whose menopausal status was unknown were classified as postmenopausal. A subgroup analysis according to strictly defined menopausal status is described in Table 5. These results translate into a 34% relative risk reduction in bone recurrence (P = .00001) and a 17% relative risk reduction in breast cancer death (P = .004) in postmenopausal women. No significant reduction was seen in recurrence outside bone. Similar benefits were observed with clodronate, compared with aminobisphosphonates such as zoledronic acid. Benefits were similar irrespective of estrogen receptor status, nodal status, and prior use of chemotherapy. No clear benefit was shown for adjuvant bisphosphonate therapy in perimenopausal or premenopausal breast cancer patients.
The reason for the difference in the effects of adjuvant bisphosphonate therapy in premenopausal and postmenopausal women is unclear. We can hypothesize that reduced bone strength caused by menopause and aging may increase susceptibility to the development of metastasis. In addition, the anticancer effects of bisphosphonates may be influenced by age- and estrogen-dependent changes to the local bone microenvironment. The ABCSG-12 study found that young women undergoing ovarian suppression may achieve a benefit similar to that of postmenopausal women. Our understanding of the exact reason for these differences is poor, and further translational research is needed in this area.
Choosing wisely
The cost-effectiveness of adjuvant bisphosphonate therapy in improving breast cancer survival has not yet been analyzed on the basis of the EBCTCG meta-analysis. Economic analysis of the ABCSG-12 study, conducted in Germany, did demonstrate that adjuvant bisphosphonate therapy is cost-effective based on clinical efficacy and quality of life measures.[51] Based on the EBCTCG meta-analysis, the number needed to treat (NNT) for adjuvant bisphosphonate therapy to prevent breast cancer mortality among postmenopausal women is only moderately favorable. (The NNT for breast cancer mortality is 53, calculated from Table 5.) However, given the high costs of metastatic breast cancer treatment, adjuvant bisphosphonate therapy will likely meet cost-effectiveness standards in most developed countries.
Summary
The use of adjuvant bisphosphonate therapy has now been demonstrated to reduce the development of bone metastasis, distant recurrence, and breast cancer mortality among postmenopausal women. These results are practice changing and will help continue to improve the ongoing care of women with breast cancer. We eagerly await the full publication of the EBCTCG meta-analysis. Current clinical practice guidelines could be revised to recommend either clodronate, 1,600 mg daily, or zoledronic acid, 4 mg every 6 months, for 3 to 5 years. The maximum duration of therapy should be 5 years at the most, given current data. We do not recommend longer dosing schedules because of concerns about long-term safety after 5 years of use, which have been reported in patients receiving treatment for osteoporosis. Bisphosphonates also have the added benefit of protecting bone health in postmenopausal women, especially in those receiving aromatase inhibitor therapy.
Conclusion
The past few years have helped provide clarity about the role of bisphosphonates in the treatment of breast cancer. Bisphosphonates continue to play a very important role in the prevention of SREs and hypercalcemia in patients with advanced disease. Zoledronic acid appears to be the most efficacious agent, although many reasonable oral and intravenous options exist for patients and physicians. We could now adopt an every-12-weeks maintenance dosing strategy for intravenous bisphosphonates in women with metastatic disease after 1 year, based on the ZOOM and OPTIMIZE-2 trial data. Therapy should continue until the patient’s health significantly declines. There are no strong data to guide treatment beyond 5 years. The EBCTCG meta-analysis has now also shown the important survival benefit of using adjuvant bisphosphonate therapy for postmenopausal breast cancer. Treatment may also be offered to premenopausal patients undergoing ovarian suppression. Adjuvant therapy leads to considerable benefit by preventing bone metastasis and improving breast cancer survival.
Future research should continue to focus on biomarker development to guide bisphosphonate therapy. Attempts to date have not been successful. Hopefully, in the coming years we will develop a useful biomarker or genomic signature to optimize patient selection for adjuvant therapy. We also need to develop improved diagnostic imaging and biomarkers to evaluate the response to treatment in patients with established bone metastasis. This will help identify bisphosphonate resistance and improve our ability to determine both the dosing frequency and the duration of necessary therapy. These advances will allow us to build on our recent successes and achievements in the treatment of metastatic breast cancer with bone metastasis.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
Acknowledgment:The authors would like to acknowledge Mark Pasetka and Sherrol Palmer and the Sunnybrook Odette Pharmacy for their valued contributions to this review.
References:
1. Lipton A. Bisphosphonates and breast carcinoma: present and future. Cancer. 2000;88:3033-7.
2. Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer. 2004;100:44-52.
3. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-45.
4. Fleisch H. Development of bisphosphonates. Breast Cancer Res. 2002;4:30-4.
5. Coleman R, Gnant M, Morgan G, Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104:1059-67.
6. Wong MH, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
7. Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314-21.
8. Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082-90.
9. Body JJ, Diel IJ, Lichinitser MR, et al. Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol. 2003;14:1399-405.
10. Heras P, Kritikos K, Hatzopoulos A, Georgopoulou AP. Efficacy of ibandronate for the treatment of skeletal events in patients with metastatic breast cancer. Eur J Cancer Care (Engl). 2009;18:653-6.
11. Kristensen B, Ejlertsen B, Groenvold M, et al. Oral clodronate in breast cancer patients with bone metastases: a randomized study. J Intern Med. 1999;246:67-74.
12. Paterson AH, Powles TJ, Kanis JA, et al. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol. 1993;11:59-65.
13. Tubiana-Hulin M, Beuzeboc P, Mauriac L, et al. Double-blinded controlled study comparing clodronate versus placebo in patients with breast cancer bone metastases. Bull Cancer. 2001;88:701-7.
14. Body JJ, Diel IJ, Lichinitzer M, et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br J Cancer. 2004;90:1133-7.
15. Diel I, Marschner N, Kindler M, et al. Continual oral versus intravenous therapy with bisphosphonates in patients with breast cancer and bone metastases. Proc Am Soc Clin Oncol. 1999;18:abstr 128a.
16. Purohit OP, Radstone CR, Anthony C, et al. A randomised double-blind comparison of intravenous pamidronate and clodronate in the hypercalcaemia of malignancy. Br J Cancer. 1995;72:1289-93.
17. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735-44.
18. Barrett-Lee P, Casbard A, Abraham J, et al. Oral ibandronic acid versus intravenous zoledronic acid in treatment of bone metastases from breast cancer: a randomised, open label, non-inferiority phase 3 trial. Lancet Oncol. 2014;15:114-22.
19. Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132-9.
20. Martin M, Bell R, Bourgeois H, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res. 2012;18:4841-9.
21. Van Poznak CH, Von Roenn JH, Temin S. American Society of Clinical Oncology clinical practice guideline update: recommendations on the role of bone-modifying agents in metastatic breast cancer. J Oncol Pract. 2011;7:117-21.
22. Coleman R, Body JJ, Aapro M, et al. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25(suppl 3):iii124-iii37.
23. Gralow JR, Biermann JS, Farooki A, et al. NCCN Task Force report: bone health in cancer care. J Natl Compr Canc Netw. 2013;11(suppl 3):S1-50; quiz S51.
24. Bonomi M, Nortilli R, Molino A, et al. Renal toxicity and osteonecrosis of the jaw in cancer patients treated with bisphosphonates: a long-term retrospective analysis. Med Oncol. 2010;27:224-9.
25. Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74:1385-93.
26. Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. N Engl J Med. 2003;349:1676-9.
27. Migliorati CA, Woo SB, Hewson I, et al. A systematic review of bisphosphonate osteonecrosis (BON) in cancer. Support Care Cancer. 2010;18:1099-106.
28. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580-7.
29. Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastasis. Ann Oncol. 2012;23:1341-7.
30. Peddi P, Lopez-Olivo MA, Pratt GF, Suarez-Almazor ME. Denosumab in patients with cancer and skeletal metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2013;39:97-104.
31. Ungprasert P, Cheungpasitporn W, Srivali N, et al. Life-threatening hypocalcemia associated with denosumab in a patient with moderate renal insufficiency. Am J Emerg Med. 2013;31:756e1-2.
32. Botteman M, Barghout V, Stephens J, et al. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol. 2006;17:1072-82.
33. Paterson A, McCloskey E, Redzepovic J, et al. Cost-effectiveness of oral clodronate compared with oral ibandronate, intravenous zoledronate or intravenous pamidronate in breast cancer patients. J Int Med Res. 2008;36:400-13.
34. De Cock E, Hutton J, Canney P, et al. Cost-effectiveness of oral ibandronate compared with intravenous (i.v.) zoledronic acid or i.v. generic pamidronate in breast cancer patients with metastatic bone disease undergoing i.v. chemotherapy. Support Care Cancer. 2005;13:975-86.
35. Stopeck A, Rader M, Henry D, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ. 2012;15:712-23.
36. Ford J, Cummins E, Sharma P, et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess (Winchester, England). 2013;17:1-386.
37. Snedecor SJ, Carter JA, Kaura S, Botteman MF. Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin Ther. 2012;34:1334-49.
38. Amadori D, Aglietta M, Alessi B, et al. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14:663-70.
39. Hortobagyi GN, Lipton A, Chew HK, et al. Efficacy and safety of continued zoledronic acid every 4 weeks versus every 12 weeks in women with bone metastases from breast cancer: results of the OPTIMIZE-2 trial. J Clin Oncol. 2014;32(suppl):abstr LBA9500.
40. Coleman R, Wright J, Houston S, et al. Randomized trial of marker-directed versus standard schedule zoledronic acid for bone metastasis from breast cancer. J Clin Oncol. 2012;30(suppl 5s):abstr 511.
41. ClinicalTrials.gov. Zoledronic acid in treating patients with metastatic breast cancer, metastatic prostate cancer, or multiple myeloma with bone involvement. 2014. Available at: https://clinicaltrials.gov/ct2/show/NCT00869206?term=70604&rank=1. Accessed October 1, 2014.
42. Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol. 2008;19:2007-11.
43. Saarto T, Vehmanen L, Virkkunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43:650-6.
44. Powles T, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 2006;8:R13.
45. Paterson AH, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13:734-42.
46. Diel IJ, Solomayer EF, Costa SD, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357-63.
47. Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631-41.
48. Coleman R, Cameron D, Dodwell D, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15:997-1006.
49. Coleman R, de Boer R, Eidtmann H, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol. 2013;24:398-405.
50. Coleman R, Gnant M, Paterson A, et al. Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer: a meta-analysis of individual patient data from randomised trials. Cancer Res. 2013;72:abstr S407.
51. Lux MP, Reichelt C, Wallwiener D, et al. Results of the Zometa cost-utility model for the German healthcare system based on the results of the ABCSG-12 study. Onkologie. 2010;33:360-8.