ADT Risks and Side Effects in Advanced Prostate Cancer: Cardiovascular and Acute Renal Injury
This article reviews recent evidence suggesting an increased risk of pneumonia, cardiovascular disease, and acute kidney injury in men treated with ADT and consider whether the incidence of such events differs with the treatment modality.
Figure 1: Cumulative Incidence Plot Depicting the Rates of AKI After Stratifying Patients According to the Type of ADT Used (no ADT vs LHRH Agonists vs Bilateral Orchiectomy)
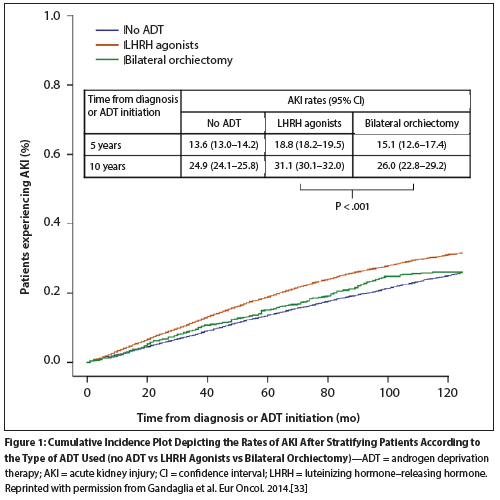
Figure 2: Staining of a Foam Cell Lesion (A,B) and a Fibrous Plaque (C,D) for CD4+ T cells (red) and Macrophages, Respectively, in Aortic Arch Lesions From 16-Week-Old Western Diet-Fed apoE-Deficient Mice
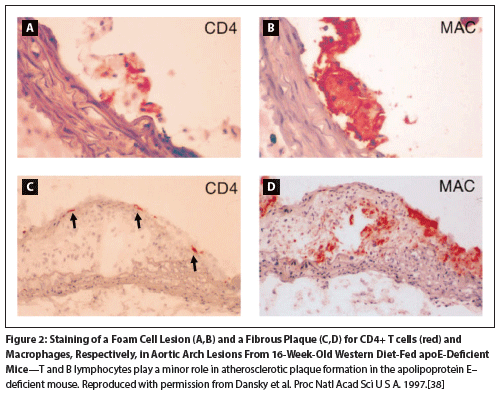
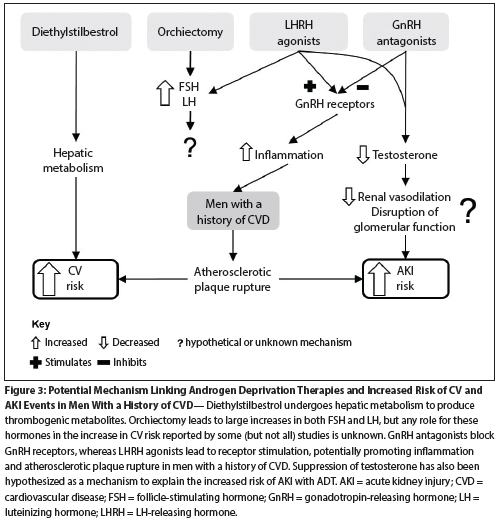
Figure 3: Potential Mechanism Linking Androgen Deprivation Therapies and Increased Risk of CV and AKI Events in Men With a History of CVD
Androgen deprivation therapy (ADT) is key to the treatment of men with advanced prostate cancer. ADT can consist of surgical (bilateral orchiectomy) or medical strategies (eg, luteinizing hormone–releasing hormone agonists or gonadotropin-releasing hormone [GnRH] antagonists). The substantial reduction of testosterone levels achieved with ADT is associated with numerous well-characterized side effects, the management of which are key to patients’ quality of life. More recently, a group of metabolic changes (dyslipidemia, hyperglycemia, others) that carry an increased risk of diabetes and cardiovascular disease have been reported in men receiving ADT. We review recent evidence suggesting an increased risk of pneumonia, cardiovascular disease, and acute kidney injury in men treated with ADT and consider whether the incidence of such events differs with the treatment modality. We discuss possible mechanisms by which such events might be mediated, including the roles of testosterone and the GnRH receptor, and consider current guidelines in light of these data.
Introduction
Because testosterone stimulates the growth of prostate cancer, androgen deprivation therapy (ADT) has often been described as the cornerstone of treatment for advanced prostate cancer. It is also recommended in the adjuvant and neoadjuvant settings for earlier-stage disease in hormone-naive patients in conjunction with radiotherapy but not with radical prostatectomy, except for the occasional patient who requires downsizing of the gland for surgical considerations.[1] ADT is achieved via surgical excision of the testes (bilateral orchiectomy), to remove the major source of testosterone production, or, more commonly, by use of drugs that disrupt signaling between the pituitary gland and the testes. The suppression of testosterone to castrate levels (traditionally defined as below 50 ng/dL or 1.73 nmol/L) is associated with numerous side effects-including hot flashes, low libido, erectile dysfunction, and decreased bone mineral density, and the management of these adverse events can be essential to patients’ quality of life (QoL).[2] More recently, other side effects have been reported, including decreased lean body mass, increased body fat, dyslipidemia, hyperglycemia, and insulin resistance.[3,4] This configuration of castration-induced changes in body composition is frequently referred to as metabolic syndrome and carries an increased risk of diabetes and cardiovascular (CV) disease (CVD).[5,6]
Recently a number of epidemiologic and population-based studies have reported the risk of pneumonia, acute kidney injury (AKI), and CVD to be higher in patients whose prostate cancer is treated with ADT. The data driving these hypotheses are described in more detail below, along with potential mechanisms of action. We also discuss current guidelines and economic considerations in the light of these data.
ADT and Risk of Pneumonia
A recent publication by Chung et al reported that among over 2,000 Taiwanese patients with prostate cancer, those who received a luteinizing hormone–releasing hormone (LHRH) agonist were almost twice as likely to be hospitalized with pneumonia during a 1-year follow-up period (hazard ratio [HR] = 1.92; 95% confidence interval [CI], 1.10–3.36) than those who did not receive an LHRH agonist.[7] The authors propose four mechanisms which may increase the risk of lung infections: morphologic and biochemical changes in the lungs, alterations in antibiotic susceptibility and microbial growth, changes in the composition of gastrointestinal microflora to allow the outgrowth of pathogenic flora, and decreased neutrophil production. To date this is the only report of an increased risk of pneumonia with ADT and thus will require confirmation in other populations, ideally as part of a prospective study. Moreover, there are no data comparing LHRH agonists vs gonadotropin-releasing hormone (GnRH) antagonists in terms of this potential new complication of treatment.
ADT and Risk of CV Events
Among the earliest medical approaches to ADT was the use of estrogens such as diethylstilbestrol (DES). It was soon apparent that DES was strongly associated with an increased risk of CV morbidity and mortality; indeed, although DES was associated with a clear reduction in prostate cancer–related deaths, overall survival was reduced due to the increase in deaths from CVD.[8,9] The increase in CV events was a consequence of the hepatic metabolism producing thrombogenic metabolites, and led to DES being discontinued as a primary therapy for the treatment of prostate cancer. Other forms of estrogen (chlorotrianisene, ethinyl estradiol) were reported to be associated with fewer CV complications, although the associated mortality rates were similar between formulations.[10]
LHRH agonists have been the most common agents used for ADT over the last 2 decades. Over the last 8 years, numerous articles have been published describing both the presence and absence of an association between ADT and increased rates of CV events. Therefore, any link between ADT and CV events is still controversial, in large part because of this wealth of conflicting evidence.
The first report identifying a possible CV risk with LHRH agonists was by Keating et al, who analyzed the Surveillance, Epidemiology and End Results (SEER) Medicare data and identified a cohort of 73,196 men with locoregional prostate cancer.[11] A significantly increased risk of coronary heart disease, myocardial infarction, and sudden cardiac death was reported for men receiving an LHRH agonist compared with those not undergoing ADT.
Further evidence supporting a link between some forms of ADT and CV events is found in several more recent retrospective epidemiologic analyses. For example, 3 studies, each comprising more than 30,000 patients, report an increased risk of CV events, including myocardial infarction, stroke, and ischemic heart disease.[12-14] However, another study investigating a database of men with prostate cancer in Canada did not find such an association.[15] Several smaller but randomized trials also found no difference in the risk of CV events between men who were treated with ADT and those who were not,[16-18] as did a meta-analysis of eight randomized clinical trials.[19]
The risk of CV events in men who have undergone bilateral orchiectomy is less clear, likely due to the small sample size given the current relative unpopularity of the procedure. Some studies found an increased risk,[12,13,20] whereas others found no association,[11,14] although there was still a link between orchiectomy and increased risk of incident diabetes.[11] It does, however, seem clear that any risk of an increase in CV events with ADT is greatest in men with a history of CVD prior to ADT treatment.[21,22] Furthermore, the risk is increased with a relatively short course of treatment. D’Amico et al showed that men aged ≥ 65 years treated with 6 to 8 months of ADT experienced shorter times to fatal myocardial infarctions compared with men who did not receive ADT (P = .017).[23] Other studies have reported that the incidence of CV events is increased within a year or less of starting ADT treatment.[24,25]
None of the epidemiology studies mentioned above considered GnRH antagonists, due to the lack of available data in the years included in the analyses. It has been reported that no difference was apparent in mean change in electrocardiographic QT abnormalities between leuprolide and the GNRH antagonist degarelix in a 1-year randomized comparative phase III trial.[26] The cardiac adverse event most frequently reported during this trial was ischemic heart disease, which occurred in 4% of patients treated with the GnRH antagonist and in 10% of patients treated with leuprolide, a difference that was not statistically significant.[27]
An analysis that pooled data from GnRH antagonist–treated patients enrolled in 9 phase II and III trials (N = 1,704) showed no increase in the baseline CV event rate once GnRH antagonist treatment was started.[28] More recently, comparative data were pooled from randomized phase III trials comparing a GnRH antagonist with LHRH agonists. Multivariate analysis described a lower incidence of CV events in men with a history of CVD at baseline (HR = 0.44; 95% CI, 0.26–0.74).[22] It is necessary to be cautious when interpreting these data, however, snce they are retrospective and based on variable trial populations; further confirmatory studies are required.
If ADT directly increases the incidence of CV events in men with prostate cancer, then it is logical to expect that less ADT treatment would be associated with fewer events. One method of achieving this is via intermittent androgen deprivation (IAD). The recent data questioning the equivalence of intermittent and continuous ADT is beyond the scope of this review, but differences in terms of overall survival have been reported. The South European Uroncological Group 9401 study found no difference in survival but this was due to the lower number of prostate cancer deaths in the continuous-treatment arm being balanced out by a higher number of CV deaths.[29] Also, the PR.7 trial reported more instances of non–prostate cancer death in the continuous-treatment arm, although this was not related specifically to a higher incidence of any one cause of death such as CV events.[30] Finally, the risk of increased cardiac disease related to hypogonadism and hormonal replacement with exogenous testosterone is also a hot topic,[31] although just as in prostate cancer, this remains a very controversial issue.
ADT and Risk of Acute Kidney Injury
Two recent reports have highlighted a further potential side effect of ADT with LHRH agonists. In 2013, Lapi et al investigated the United Kingdom Clinical Practice Research Datalink and identified 10,250 men newly diagnosed with nonmetastatic prostate cancer.[32] A nested case-control approach was used to match AKI cases with up to 20 randomly selected controls by age, calendar year of prostate cancer diagnosis, and duration of follow-up.
As would be expected, men experiencing AKI were more likely to have reported several baseline factors known to be related an to increased risk of AKI, including excessive alcohol use, smoking, diabetes, and CVD. In terms of factors related to prostate cancer, metastatic disease, prostatectomy, and chemotherapy were associated with a higher rate of AKI at baseline. The key finding was that ADT use within the previous 90 days was associated with a significantly increased risk of AKI compared with that in men never exposed to ADT (odds ratio [OR] = 2.48; 95%CI, 1.61–3.82). When the cohort was segregated by type of ADT received, then the use of LHRH agonists, estrogen, combined androgen blockade, and other combination therapies all significantly increased the risk of AKI. Use of oral antiandrogens and bilateral orchiectomy were associated with an increased risk of AKI, but this was not significant.
A second study has also investigated the incidence of AKI during ADT treatment. Gandaglia et al utilized the SEER Medicare database to identify a cohort of 29,408 men with nonmetastatic prostate cancer.[33] Propensity-score matching was used to reduce the inherent bias due to differences between patients who were treated with ADT and those who were not. For patients receiving ADT, the estimated 10-year AKI rate was significantly higher (30.7%, compared with 24.9% for ADT-naive patients), and the incidence rates over 5 years and 10 years were also higher in men treated with LHRH agonists than in those who underwent bilateral orchiectomy (Figure 1). Multivariate analysis of the type of ADT received confirmed a significantly higher risk of AKI in men treated with LHRH agonists (HR = 1.24; 95% CI, 1.18–1.31) but not in those treated with bilateral orchiectomy (HR = 1.11; 95% CI, 0.96–1.29).[33] Treatment with LHRH agonists, but not bilateral orchiectomy, was also associated with chronic kidney failure, which the authors noted may be related to protracted kidney injury associated with long-term LHRH agonist use.
When the duration of ADT treatment was split into tertiles, the highest risk of AKI was within the first third of the total duration of ADT treatment (< 386 days). The OR remained significant over the second and final thirds of ADT treatment but decreased in each period.[32] This suggests that the greatest risk of an AKI is within approximately the first year of receiving ADT, a timeframe similar to that suggested for the risk of a CV event. It would be interesting to know whether dividing the treatment period into shorter units of time would further define the highest period of risk after initiation of ADT.
Neither of the studies published to date assessed the potential risk of AKI with a GnRH antagonist. Unfortunately, randomized phase III trials comparing GnRH antagonists with LHRH agonists did not specifically report AKI as an adverse event. Consequently, there are no direct data available, and the closest outcome measure reported from clinical trials was urinary tract events. In this category, fewer events were reported with a GnRH antagonist compared with LHRH agonists (HR = 0.61; 95% CI, 0.48–0.78).[34] However, these data do not inform as to the incidence of AKI with GnRH antagonists; this needs to be confirmed by future studies directly addressing the issue.
Mechanisms of Action
When considering how treatment with ADT induces side effects, it is logical to first consider the impact of castrate testosterone levels on the systems in question. Lack of testosterone has a number of effects that may be linked to AKI.
First, metabolic changes associated with ADT, such as dyslipidemia and hyperglycemia, can lead to expansion and thickening of the interstitial tubular membrane, which in turn disrupts glomerular function.[35] This effect could be augmented by the absence of the protective effect of testosterone on peripheral circulation shown in a preclinical model, whereby testosterone induces vasodilation of renal vessels.[36] However, testosterone has been reported to have a multitude of effects on the circulatory system, including cardioprotection, both positive and negative effects on atherosclerosis, and a role in both vasodilation and vasoconstriction. Furthermore, mechanisms directly or solely contingent upon testosterone suppression cannot explain the difference in AKI between patients treated with LHRH agonists and those treated with orchiectomy. Thus, at this time, proposing testosterone suppression as the cause of AKI is problematic.
Although possibly coincidental, it is noteworthy that the timeframes of CV and AKI events recorded during ADT are similar. Also, both adverse effects are seen with LHRH agonists but not (or to a lesser degree) with antiandrogens and bilateral orchiectomy, suggesting a potential drug class effect.
Since it has also been reported that patients with baseline CV comorbidities have an increased risk of AKI compared with those without CV diseases at baseline (HR = 1.23; 95% CI, 1.13–1.33; P < .001),[37] a single mechanism that could explain both AKI and CV events would be attractive. Studies investigating both these events have speculated on the role of GnRH receptors on T helper lymphocytes and a potential link to atherosclerotic plaque rupture. Studies in mice have shown the presence of CD4+ T cells in aortic arch foam cell lesions and in the subendothelium of fibrous plaques (Figure 2).[38] In humans, activation of GnRH receptors has been demonstrated to promote proliferation, differentiation to the Th1 phenotype, and production of interferon-γ.[39] Th1 cells drive a pro-inflammatory environment that can ultimately lead to destabilization of atherosclerotic plaques; subsequent plaque rupture may lead to downstream thrombotic complications, including myocardial infarction and ischemic injury in various organs. This is currently a creditable hypothesis, but further studies are required to fully define the mechanism(s) involved (Figure 3).
CV and Kidney Safety Considerations in Current Guidelines
Based on the available evidence, the US Food and Drug Administration (FDA) issued a drug safety communication requiring new safety information about the risk of diabetes and certain CV diseases with ADT, and a consensus paper was published by the American Heart Association, the American Cancer Society, and the American Urological Association.[40] Current National Comprehensive Cancer Network guidelines recommend screening for CV disorders and intervening to prevent or treat CVD, considering that CVD is relatively common in the general population and increases with age, as does the incidence of prostate cancer. However, the guidelines also note that it is unclear whether screening, prevention, and treatment strategies for CVD should differ between men receiving ADT and those in the general population.
In Europe, the latest European Association of Urology guidelines judge the available data on CV mortality to be inconsistent and make no specific recommendations in relation to ADT but note that general improvements in health (including CV fitness) can be gained by adoption of nonspecific measures, including weight loss, increased exercise, improved nutrition, and smoking cessation. As of late 2014, the authors are uncertain whether existing guidelines should be updated. However, expert panels must keep a close eye on this area. Currently, footnotes should be considered in the guidelines to apprise the practicing clinician of these emerging concepts of toxicity and risk.
Considering the debate surrounding the data, the absence of clear guidelines, and the lack of prospective trials (which are overlapping issues), it is unlikely that these data, which are also only very recent, have yet brought about changes in clinical practice.
Economic Considerations
In purely economic terms, bilateral orchiectomy is the most cost-effective form of ADT, while combined androgen blockade is the most expensive option, associated with a high cost yet only relatively small additional treatment benefits.[41] When considering therapy with LHRH agonists, early treatment is associated with higher costs and greater frequency of treatment-related adverse effects.[41] The greatest QoL gains at the lowest cost with LHRH agonists may be obtained by starting ADT when symptoms from distant metastases have occurred. However, deferred treatment risks the development of hormone independence in the tumor, serious complications such as spinal cord compression, patient and family anxiety, and reduced QoL from concern about nonintervention in the face of rising prostate-specific antigen (PSA) levels. If a substantial tumor response is achieved once ADT is started, then IAD might be a practical way to reduce treatment costs as well as increase QoL.
However, with recent data suggesting that IAD treatment may not be as effective as continuous ADT in men with M1 disease,[42] it is possible that bilateral orchiectomy should be considered for more men than it has been in recent years.
In terms of differences in cost between medical ADTs, two models report use of a GnRH antagonist to be a cost-effective option compared with an LHRH agonist (with or without an antiandrogen). From a US payer’s perspective, GnRH antagonist use was found to be more cost-effective than leuprolide,[43] and in a UK-based analysis was dominant compared with leuprolide use in the overall patient population; the greatest benefit was in the subgroup of men with PSA levels > 20 ng/mL.[44] However, a previous analysis reported that the incremental cost-effectiveness ratio per quality-adjusted life-year (QALY) gained with GnRH antagonists was above the threshold required in the United Kingdom.[45] The impact of the latest safety data relating to potential differences in the adverse effects of ADT on treatment costs requires analysis beyond the expertise and scope of this review, and the outcomes of such analyses are awaited with interest.
Summary
Aside from the well-known side effects of ADT associated with the suppression of testosterone, ADT appears to be associated with an increased risk of CV and AKI events. Interestingly, this risk may differ depending on the method used to suppress testosterone. A possible mechanism to explain differences between LHRH agonists, bilateral orchiectomy, and GnRH antagonists in the rates of these two types of adverse events is the disruption of atherosclerotic plaques. Preclinical studies evaluating potential mechanisms by which these events may be mediated are required, along with further studies evaluating patients prospectively enrolled in randomized trials to confirm the risk of CV and AKI events with ADT.
Acknowledgements:Medical writing assistance, funded by Ferring Pharmaceuticals, was provided by Bioscript Medical. However, the sponsor had no input into the content of the review and both authors contributed equally to the full scientific content of this manuscript. Furthermore, Ferring Pharmaceuticals did not review any aspects of the manuscript before, during, or after drafting or publication. The authors received no compensation from any source for work on this manuscript.
Financial Disclosure:Dr. Crawford is an advisor, meeting participant, and paid consultant for Ferring, and receives research funding from Ferring; his wife is a Ferring employee. Dr. Moul is an advisor and meeting participant for Ferring, and a consultant for Tolmar Pharmaceuticals.
References:
1. National Comprehensive Cancer Network. NCCN guidelines for prostate cancer. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed December 30, 2014.
2. Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2014 Aug 2. [Epub ahead of print]
3. Hakimian P, Blute M, Jr, Kashanian J, et al. Metabolic and cardiovascular effects of androgen deprivation therapy. BJU Int. 2008;102:1509-14.
4. Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2009;181:1998-2006.
5. Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29:534-9.
6. Nobes JP, Langley SE, Laing RW. Metabolic syndrome and prostate cancer: a review. Clin Oncol (R Coll Radiol). 2009;21:183-91.
7. Chung SD, Liu SP, Lin HC, Wang LH. Increased risk of pneumonia in patients receiving gonadotropin-releasing hormone agonists for prostate cancer. PLoS One. 2014;9:e101254.
8. Bailar JC 3rd, Byar DP. Estrogen treatment for cancer of the prostate. Early results with 3 doses of diethylstilbestrol and placebo. Cancer. 1970;26:257-61.
9. Byar DP. Proceedings: The Veterans Administration Cooperative Urological Research Group’s studies of cancer of the prostate. Cancer. 1973;32:1126-30.
10. Morales A, Pujari B. The choice of estrogen preparations in the treatment of prostatic cancer. Can Med Assoc J. 1975;113:865-7.
11. Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448-56.
12. Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39-46.
13. Van Hemelrijck M, Garmo H, Holmberg L, et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the Population-Based PCBaSe Sweden. J Clin Oncol. 2010;28:3448-56.
14. Jespersen CG, Norgaard M, Borre M. Androgen-deprivation therapy in treatment of prostate cancer and risk of myocardial infarction and stroke: a nationwide Danish population-based cohort study. Eur Urol. 2014;65:704-9.
15. Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452-8.
16. Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009;27:92-9.
17. Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066-73.
18. Wilcox C, Kautto A, Steigler A, Denham JW. Androgen deprivation therapy for prostate cancer does not increase cardiovascular mortality in the long term. Oncology. 2012;82:56-8.
19. Nguyen PL, Je Y, Schutz FA, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306:2359-66.
20. Azoulay L, Yin H, Benayoun S, et al. Androgen-deprivation therapy and the risk of stroke in patients with prostate cancer. Eur Urol. 2011;60:1244-50.
21. Nanda A, Chen MH, Braccioforte MH, et al. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302:866-73.
22. Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565-73.
23. D’Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420-5.
24. Kintzel PE, Chase SL, Schultz LM, O’Rourke TJ. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy. 2008;28:1511-22.
25. Ziehr DR, Chen MH, Zhang D, et al. Association of androgen deprivation therapy with excess cardiac-specific mortality in men with prostate cancer. BJU Int. 2014 Aug 15. [Epub ahead of print]
26. Smith MR, Klotz L, Persson BE, et al. Cardiovascular safety of degarelix: results from a 12-month, comparative, randomized, open label, parallel group phase III trial in patients with prostate cancer. J Urol. 2010;184:2313-9.
27. Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531-8.
28. Smith MR, Klotz L, van der Meulen E, et al. Gonadotropin-releasing hormone blockers and cardiovascular disease risk: analysis of prospective clinical trials of degarelix. J Urol. 2011;186:1835-42.
29. Calais da Silva FE, Bono AV, Whelan P, et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009;55:1269-77.
30. Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895-903.
31. Vigen R, O’Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-36.
32. Lapi F, Azoulay L, Niazi MT, et al. Androgen deprivation therapy and risk of acute kidney injury in patients with prostate cancer. JAMA. 2013;310:289-96.
33. Gandaglia G, Sun M, Hu JC, et al. Gonadotropin-releasing hormone agonists and acute kidney injury in patients with prostate cancer. Eur Urol. 2014;66:666-72.
34. Klotz L, Miller K, Crawford ED, et al. Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists. Eur Urol. 2014;66:1101-8.
35. Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498-509.
36. Molinari C, Battaglia A, Grossini E, et al. Effects of insulin on coronary blood flow in anesthetized pigs. J Vasc Res. 2002;39:504-13.
37. Gandaglia G, Sun M, Briganti A, Karakiewicz PI. Reply to E. David Crawford and Bo-Eric Persson’s Letter to the Editor re: Gandaglia G, Sun M, Hu JC, et al. Gonadotropin-releasing hormone agonists and acute kidney injury in patients with prostate cancer. Eur Urol. In press. http://dx.doi.org/10.1016/j.eururo.2014.01.026. Eur Urol. 2014;66:e36-7.
38. Dansky HM, Charlton SA, Harper MM, Smith JD. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA. 1997;94:4642-6.
39. Tanriverdi F, Gonzalez-Martinez D, Hu Y, et al. GnRH-I and GnRH-II have differential modulatory effects on human peripheral blood mononuclear cell proliferation and interleukin-2 receptor gamma-chain mRNA expression in healthy males. Clin Exp Immunol. 2005;142:103-10.
40. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-40.
41. Bayoumi AM, Brown AD, Garber AM. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst. 2000;92:1731-9.
42. Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314-25.
43. Hatoum HT, Crawford ED, Nielsen SK, et al. Cost-effectiveness analysis comparing degarelix with leuprolide in hormonal therapy for patients with locally advanced prostate cancer. Expert Rev Pharmacoecon Outcomes Res. 2013;13:261-70.
44. Lee D, Porter J, Gladwell D, et al. A cost-utility analysis of degarelix in the treatment of advanced hormone-dependent prostate cancer in the United Kingdom. J Med Econ. 2014;17:233-47.
45. Lu L, Peters J, Roome C, Stein K. Cost-effectiveness analysis of degarelix for advanced hormone-dependent prostate cancer. BJU Int. 2012;109:1183-92.