A Role for Hepatic Metastasectomy in Stage IV Melanoma and Breast Cancer: Reestablishing the Surgical Modality
This review summarizes the existing literature that addresses the topic of metastasectomy in patients with melanoma and breast cancer.
Historically, liver-related metastases associated with melanoma or breast cancer have portended a poor prognosis. Many affected patients are not considered for surgical resection based on the extent and multifocal nature of their disease. For this patient population, treatment includes systemic and/or regional therapy, local destruction (ablation/radiation), and embolization. Despite the best therapeutic regimens, prognosis remains poor. Advances in surgical technique and postoperative care have led to a resurgence in the use of metastasectomy, most notably seen in patients with colorectal-related liver metastases. With the potential for therapeutic durability and a small chance of cure, surgical resection may offer improved survival compared to other therapeutic modalities. This review summarizes the existing literature that addresses the topic of metastasectomy in patients with melanoma and breast cancer.
Introduction
Advances in perioperative planning, surgical technique, and postoperative care over the past two decades have allowed the indications for hepatic resection to be potentially broadened to include histologies and/or disease burdens previously felt to be contraindications for this treatment modality. This trend has been seen most notably with respect to resection of colorectal-related liver metastases.[1,2] Historically, colorectal liver metastases have been treated with palliative chemotherapy. The improvements seen with surgical resection have made this the gold standard for treatment; expected 5-year survival rates are 50% to 65%, and resection offers the only potential for cure.[3] Hepatic resection for neuroendocrine-related liver metastases is likewise now accepted as appropriate.[4] However, while retrospective reports have demonstrated long-term survival in some highly selected patients with other histologies, including melanoma and breast cancer, the application of hepatectomy in these patients remains controversial.
There are a number of arguments against the use of hepatectomy in the management of metastatic disease. First and foremost is the lack of randomized data to support its utilization, even in patients with neuroendocrine and colorectal histologies. Hepatectomy is associated with severe morbidity rates-20% to 30%-and with 90-day mortality rates of 1% to 5%.[5] Disease recurs in the overwhelming majority of patients following hepatectomy, and the prospect of recurrence exceeds 80% even in the most favorable of histologies.[6] However, similar statements can be made to argue against surgical resection of pancreatic cancer, gastric cancer, esophageal cancer, and primary liver cancer. Ultimately, patients and their oncologists are drawn to the small to modest possibilities of cure that are associated with hepatectomy and other complicated oncologic resections in the face of what would be a near-zero possibility of cure without resection. For young, healthy patients, it is a relatively easy decision, although for older and/or infirm patients the decisions are more complex.
For the vast majority of patients with liver metastases who are not candidates for surgical resection, treatment options include systemic and/or regional therapy, local destruction (ablation/radiation), and embolization. Systemic therapy options have evolved, and there are now a multitude of agents available for metastatic disease. However, response rates have been low and the effect on overall survival is still unclear. Regional therapy, including the use of isolated hepatic artery treatment, has been shown to achieve partial remission, although it has no significant effect on long-term survival. Lastly, embolization techniques have been promising, with reports showing better survival rates than those associated with the best chemotherapy, with minimal toxicity.[7] Still, even this therapy is palliative at best; surgical resection offers the only real opportunity for cure.
The aim of this review is to present an analysis of the existing literature that addresses the topic of metastasectomy in patients with melanoma and breast cancer. Despite improvements in systemic chemotherapy for these diseases, the prognosis for metastasized disease in both cases remains poor.[8,9] With proper patient selection, the added modality of surgical resection may allow for improved survival and, as with colorectal and neuroendocrine cancers, may be the only possibility of cure.
Melanoma
With an estimated 68,130 cases diagnosed in 2010, cutaneous melanoma is approximately 25 times more common than ocular melanoma.[16] While each form has the potential to metastasize to the liver, the rates at which they do so are distinctly different. The difference in the metastatic pattern is likely a direct result of the differing tumor biology of the two cancers. Up to 95% of patients with stage IV ocular melanoma develop hepatic metastasis.[17] In contrast, this occurs in only 10% to 20% of patients with stage IV cutaneous melanoma, with the majority of metastases occurring primarily in distant lymph nodes or soft tissue.[18,19] Ocular melanoma, on the other hand, exhibits latency, with metastases appearing many years following removal of the primary disease.[20] The liver is the sole site of metastasis in 60% to 80% of cases, with characteristic intrahepatic multiplicity.[21,22] Historically, prognosis is poor in patients with hepatic metastases of ocular melanoma, with life expectancy less than 6 months.
Overview of nonsurgical approaches
The presentation of stage IV melanoma with isolated liver metastasis has a historically poor prognosis, with a median survival of 4 to 6 months.[10] Therapy for liver metastasis is limited: options include systemic and regional therapy, local destruction (ablation/radiation), and surgical resection.
Systemic options have historically included dacarbazine, temozolomide (Temodar), interleukin-2, paclitaxel, cisplatin, and carboplatin.[11,12] Response rates have been low, and the effect on overall survival unclear at best. Within the past year, highly publicized reports presented at the annual meeting of the American Society of Clinical Oncology (ASCO) have suggested improvements in overall survival with two novel targeted agents-vemurafenib (PLX4032; Zelboraf) and ipilimumab (Yervoy).[8,9] In one trial (BRIM-3), vemurafenib was shown to improve response and survival (overall and progression-free) as a single agent compared with standard treatment (dacarbazine). The results were felt to be so striking that the trial was halted early (with a median follow-up of just over 3 months) to allow cross-over of the control arm. The efficacy of ipilimumab was confirmed in a second trial, also reported at ASCO's 2011 annual meeting.[13]
Regional therapies, including isolated hepatic perfusion, have shown promising results in the treatment of unresectable hepatic metastases. Historically, hepatic perfusion has been associated with significant morbidity due to the complex nature of the technique; however, recent advances using percutaneous techniques have allowed for a resurgence in the evaluation of this method. Hepatic artery treatment with fotemustine has been promising, producing a 40% response rate and overall survival of 14 months.[14] Phase I studies using hepatic arterial melphalan infusion have shown the ability to achieve partial remission, although no significant effect on long-term survival has been demonstrated.[15] Despite the benefits of this modality, no durable response has been achieved. Surgical resection in patients with isolated hepatic metastasis, while rarely performed, appears to offer the only real opportunity for cure.
Hepatectomy for metastatic melanoma: a review of the literature
FIGURE 1
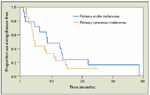
Disease-Free Survival in Patients With Primary Ocular or Primary Cutaneous Melanoma
The existing literature on the topic of hepatectomy for metastatic melanoma consists of small case reports of what appear to be highly selected patients. In a notable review of the prospectively maintained melanoma database at the John Wayne Cancer Institute, Rose et al analyzed 1750 patients who underwent treatment for melanoma metastases to the liver between 1971 and 1999.[23] Meticulous selection of patients resulted in only 34 patients (2%) who met criteria for attempted surgical resection. Ten patients were deemed not resectable due to extent of disease at the time of surgery. Of the 24 remaining, 18 (75%) were rendered free of disease following resection. The median overall survival for the 18 patients with curative resection was 38 months. Median overall survival and median disease-free survival for the 24 patients with attempted resection were 28 months and 12 months, respectively, compared with a median survival of 4 months in the 10 patients who had exploration only. Prolonged disease-free interval, complete tumor resection, and lower tumor burden were all associated with improved outcomes.
In a report by Adam et al consisting of 1452 patients who underwent hepatic resection for noncolorectal, nonendocrine liver metastases, a subset of 44 patients with cutaneous melanoma liver metastases was described.[24] The 5-year survival was 22%. Analysis of factors associated with poor prognosis revealed that these include age greater than 60 years, disease-free interval less than 12 months, extrahepatic metastases, R2 resection, and major hepatectomy. Similarly, Pawlik et al described their experience of 40 patients with hepatic melanoma, the primary tumor being cutaneous in 24 patients and ocular in 16 patients.[25] Median disease-free interval was 63 months. Recurrence developed in 75% of patients after resection, with a median time to recurrence of 8.8 months for the ocular subset and 4.7 months for the cutaneous subset. The 5-year survival for patients with primary ocular melanoma was 20.5%; there were no 5-year survivors in the cutaneous melanoma group (Figure 1).
TABLE 1

Data Review of Modern Liver Metastectomy Series for Uveal Melanoma
Much as with cutaneous melanoma, multiple reports have documented the benefit of surgical resection for hepatic metastases of ocular melanoma.[26-28] Rivoire et al followed 602 patients between 1983 and 1996 who were treated for ocular melanoma (Table 1).[26] Using ultrasound, patients were evaluated to assess for development of hepatic metastases. Liver metastases developed in 63 patients (10.5%) following ocular treatment, with a median disease-free interval of 29 months. Twenty-eight patients (4.7%) met criteria for attempted surgical resection, with 14 patients undergoing R0 surgery. Median survival was 25 months for patients with R0 surgery, 16 months for R2 resection (14 patients), and 11 months for the 35 patients treated with palliative chemotherapy. When the data were subjected to univariate analysis, age < 70 years and completeness of surgical resection (R0) were associated with improved outcomes.
A series by Mariani et al retrospectively reviewed 3873 patients with ocular melanoma between 1991 and 2007.[27] Hepatic metastases developed in 798 patients (21%), with a median time interval between ocular tumor diagnosis and treatment of hepatic metastases of 68 months. Of the patients who developed liver metastases, 255 (6.6%) underwent attempted surgical resection. R0, R1, and R2 surgery was performed in 76, 22, and 157 patients, respectively. Median overall survival was 27 months for R0 surgery, 17 months for R1 surgery, and 11 months for R2 surgery. Multivariate analysis revealed that completeness of surgical resection (R0 surgery), number of metastases resected (< 4), and disease-free interval following primary tumor diagnosis > 24 months were associated with improved outcomes.
FIGURE 2
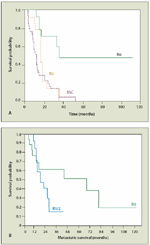
Survival According to Treatment of Uveal Melanoma–Associated Liver Metastases
More recently, Frenkel et al evaluated 558 patients with ocular melanoma, of whom 74 (13%) developed metastases.[28] The median disease-free interval was 35 months from initial diagnosis. Only 35 patients underwent hepatectomy. Median survival for patients with fewer than 5 metastases was 55.2 months. Patients with > 5 metastases or miliary metastases had a median survival of 25.6 months. The survival of patients with an R0 resection was four times longer than the survival of those with an R1 or R2 resection (Figure 2).
Selection of patients for resection
Selection of the optimal patient for resection of melanoma liver metastases is based on multiple factors. A long disease-free interval, the ability to achieve an R0 resection, low tumor multiplicity, and no or limited extrahepatic disease increase the durability of surgical resection. This point was more eloquently made by Coit et al,[29] who in referring to metastatic melanoma stated: “regardless of the extent of the operative procedure, resection of metastases in patients whose disease recurs early after the treatment of the primary tumor, in those who present with multiple lesions, and in those who present with disease that cannot be completely resected will only rarely be associated with subsequent long-term survival.”
FIGURE 3
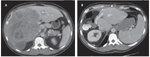
Representative abdominal CT scan of a 48-year-old-man with isolated metastatic melanoma of unknown primary
It would be easy to be nihilistic if not for the real prospect of long-term survival seen in practices where aggressive approaches are taken. Although the analogy is imperfect, the concept of “variable interval reinforcements” is likely operative in such practices, with the occasional successes reinforcing enthusiasm for aggressive treatments. Figure 3 depicts one example of such a success-a 48-year-old male who presented to our institution for the treatment of metastatic melanoma. The patient initially presented with fatigue and anemia (hemoglobin level of 3.5 g/dL). Colonoscopy revealed an ileal lesion with biopsy-proven melanoma of unknown primary. Evaluation included a computed tomography (CT)/positron emission tomography (PET) scan, which identified an additional lesion in the liver. The patient underwent a small bowel resection from which he recovered unremarkably; he then started interleukin-2 therapy. Two years later he developed rapid progression of disease isolated to the right liver and was at that time referred for possible liver resection. The patient underwent an uneventful right hepatectomy and is currently disease free 6 years later.
Breast Cancer
Advances in locoregional and systemic therapy allow for a potential cure of patients with early-stage breast cancer, but for patients with metastatic breast cancer the treatment is often one of palliation. Approximately 50% of breast cancer patients develop distant metastases; these commonly involve the bones, liver, lung, and/or brain.[30,31] The prognosis for such patients is poor, with a 5-year survival of 23.4%, as demonstrated by Howlader et al in their review of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database.[32] As many as 50% of patients with stage IV disease develop liver metastases, with an associated median survival ranging from 3 to 15 months.[33-35] Liver metastases are present in 15% of patients with newly diagnosed metastatic breast cancer, and the liver is the only site of distant disease in one third of these patients.[36,37] Due to the frequency of chemoresistance and the negative hormone receptor status of most liver metastases, the response to systemic therapy is often limited, such that durable complete responses are infrequent.[38]
Significant progress has been made in the multimodality treatment of patients with breast cancer; advances include the use of more effective chemotherapy (anthracyclines and taxanes), antihormonal therapy (aromatase inhibitors), and directed biologic agents (trastzumab [Herceptin]). Nonetheless, the development of distant metastasis continues to be associated with a very poor prognosis.[39] Despite the limitations of systemic therapy, local treatment options for managing hepatic metastases are still rarely considered. The reasons for this include the common occurrence of extrahepatic metastases,[40] which have traditionally been a contraindication to hepatic resection, and the overall poor prognosis of patients with breast cancer–related liver metastases.
Hepatectomy for metastatic breast cancer: a review of the literature
TABLE 2

Data Review of Modern Liver Metastasectomy Series for Breast Cancer
A number of reports have been published evaluating the role of hepatic resection for metastatic breast cancer in highly selected patients. Most recently, Chua et al authored a review of hepatic resection for metastatic breast cancer, evaluating published studies reporting outcomes of breast cancer–related hepatectomy between January 2000 and January 2010; these studies were analyzed for safety, efficacy, and prognostic factors associated with survival.[41] In total, 19 studies were reviewed, in which 533 patients were identified who underwent hepatectomy for breast cancer–related metastasis. The median time from primary tumor to development of liver metastasis was 40 months (range, 23 to 77 months). Median overall survival was 40 months, with a median 5-year survival of 40% (range, 21% to 80%). The median postoperative mortality rate was 0% (range, 0% to 6%). The prognostic factors of R2 surgical resection and hormone-refractory disease were associated with worse outcomes.
Much as with melanoma, the majority of studies of breast cancer–related hepatic resection are relatively small. The largest series, by Adam et al, reviewed 85 consecutive patients with breast cancer liver metastasis treated with hepatic resection between 1984 and 2004.[42] In this study, because of improved outcomes following hepatic resection, no mortality was seen within 60 days of surgery. R0 surgery was achieved in 66 patients and R1 surgery in 15 patients. Median survival was 32 months, and 5-year overall survival was 37%. For 59 patients who developed metastatic disease following hepatic resection, the median time to recurrence was 10 months. Of this subgroup, 31 patients developed extrahepatic or intra- and extrahepatic recurrences. For patients with disease limited to the liver (n = 12), repeat hepatectomy was performed. Multivariate analysis revealed that factors associated with worse outcomes included poor response to preoperative chemotherapy (5-year overall survival, 0% to 10%), R2 surgery (5-year overall survival, 10%), and inability to undergo repeat hepatectomy due to extrahepatic disease (5-year overall survival, 29%).
Elias et al reported similar findings in their experience with hepatic resection in 54 patients with metastatic breast cancer between 1986 and 2001.[43] Major hepatic resection (> 3 segments) was performed in 32 patients, with 20 patients undergoing a right hepatectomy. R0 surgery was achieved in 44 patients. Median survival was 34.3 months, and 5-year overall survival was 34%-results comparable to those of Adam et al. Disease-free interval for liver recurrence was 16 months, with only 5 of 30 recurrences occurring in bone, lung, or brain first. Multivariate analysis revealed that only hormone receptor status (P = .03) influenced survival, with the risk of death increased 3.5-fold when status was negative.
Additional studies by Hoffmann and Thelen, though small, have shown a similar benefit for hepatic resection for metastatic breast cancer.[44,45] With minimal surgical mortality, hepatic resection achieved a 5-year survival of 42% to 48% in selected patients with stage IV breast cancer. Poor prognostic factors included positive resection margins and disease-free interval between the treatment of the primary tumor and the diagnosis of hepatic metastasis < 1 year (Table 2).
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Carboplatin
Cisplatin
Dacarbazine
Fotemustine
Interleukin-2
Ipilimumab (Yervoy)
Paclitaxel
Temozolomide (Temodar)
Trastuzumab (Herceptin)
Vemurafenib (PLX4032; Zelboraf)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Conclusion
Surgical indications for patients with stage IV disease processes are expanding. With advances in surgical technique and perioperative care, the morbidity and mortality of patients undergoing hepatic resection have declined dramatically over the last two decades. As minimally invasive techniques become more widespread, the associated surgical morbidity can be anticipated to diminish further. These improvements have allowed surgery to be reincorporated into the multimodal treatment of advanced disease processes, including metastatic melanoma and breast cancer.
Surgical resection of hepatic metastases in the setting of stage IV melanoma and breast cancer has been clearly associated with long-term survival. Prognostic factors including completeness of surgical resection, prolonged disease-free interval, low multiplicity of metastases, and no or limited extrahepatic disease give insight into the patients who would be most likely to benefit from surgical resection. Improving the methods by which we stratify patients in whom surgical resection will be an optimal treatment is imperative. As systemic therapies improve, an increased role for surgical resection will follow in treating patients with previously untreatable disease.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. Fong Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J Clin. 1999;49:231-55.
2. Fuhrman GM, Curley SA, Hohn DC, Roh MS. Improved survival after resection of colorectal liver metastases. Ann Surg Oncol. 1995;2:537-41.
3. Adam R, Pascal G, Azoulay D, et al. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871-83; discussion 83-4.
4. Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432-45.
5. Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46.
6. Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-66.
7. Kennedy AS, Nutting C, Jakobs T, et al. A first report of radioembolization for hepatic metastases from ocular melanoma. Cancer Invest. 2009;27:682-90.
8. Wolchok JD, Thomas L, et al. Phase III randomized study of ipilimumab plus dacarbazine vs DTIC alone as first-line treatment in patient with unresectable stage III or IV melanoma. ASCO Annual Meeting. 2011;Abstract LBA5.
9. Chapman PB, Robert C, et al. Phase III randomized, open-label multicenter trial (BRIM3) comparing BRAF inhibitor vemurafenib with dacarbazine (DTIC) in patients with V600E BRAF-mutated melanoma. ASCO Annual Meeting. 2011;Abstract LBA4.
10. Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181:193-201.
11. Garbe C, Eigentler TK, Keilholz U, et al. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16:5-24.
12. Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823-30.
13. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-26.
14. Leyvraz S, Spataro V, Bauer J, et al. Treatment of ocular melanoma metastatic to the liver by hepatic arterial chemotherapy. J Clin Oncol. 1997;15:2589-95.
15. Pingpank JF, Libutti SK, Chang R, et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol. 2005;23:3465-74.
16. Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology. 2003;110:956-61.
17. Becker JC, Terheyden P, Kampgen E, et al. Treatment of disseminated ocular melanoma with sequential fotemustine, interferon alpha, and interleukin 2. Br J Cancer. 2002;87:840-5.
18. Leiter U, Meier F, Schittek B Garbe C. The natural course of cutaneous melanoma. J Surg Oncol. 2004;86:172-8.
19. Cohn-Cedermark G, Mansson-Brahme E, Rutqvist LE, et al. Metastatic patterns, clinical outcome, and malignant phenotype in malignant cutaneous melanoma. Acta Oncologica. 1999;38:549-57.
20. Crowley NJ, Seigler HF. Late recurrence of malignant melanoma. Analysis of 168 patients. Ann Surg. 1990;212:173-7.
21. Rajpal S, Moore R, Karakousis CP. Survival in metastatic ocular melanoma. Cancer. 1983;52:334-6.
22. Rietschel P, Panageas KS, Hanlon C, et al. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005;23:8076-80.
23. Rose DM, Essner R, Hughes TM, et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg. 2001;136:950-5.
24. Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244:524-35.
25. Pawlik TM, Zorzi D, Abdalla EK, et al. Hepatic resection for metastatic melanoma: distinct patterns of recurrence and prognosis for ocular versus cutaneous disease. Ann Surg Oncol. 2006;13:712-20.
26. Rivoire M, Kodjikian L, Baldo S, et al. Treatment of liver metastases from uveal melanoma. Ann Surg Oncol. 2005;12:422-8.
27. Mariani P, Piperno-Neumann S, Servois V, et al. Surgical management of liver metastases from uveal melanoma: 16 years’ experience at the Institut Curie. Eur J Surg Oncol. 2009;35:1192-7.
28.Frenkel S, Nir I, Hendler K, et al. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br J Ophthalmol. 2009;93:1042-6.
29. Allen PJ, Coit DG. The surgical management of metastatic melanoma. Ann Surg Oncol. 2002;9:762-70.
30. Hoe AL, Royle GT, Taylor I. Breast liver metastases-incidence, diagnosis and outcome. J Royal Soc Med. 1991;84:714-6.
31. Zinser JW, Hortobagyi GN, Buzdar AU, et al. Clinical course of breast cancer patients with liver metastases. J Clin Oncol. 1987;5:773-82.
32. Howlader N, Krapcho M, Neyman N, et al (editors). SEER Cancer Statistics Review, 1975-2008, National Cancer Institute. Bethesda, MD. Available from: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011.
33. Goldhirsch A, Gelber RD, Castiglione M. Relapse of breast cancer after adjuvant treatment in premenopausal and perimenopausal women: patterns and prognoses. J Clin Oncol. 1988;6:89-97.
34. O’Reilly SM, Richards MA, Rubens RD. Liver metastases from breast cancer: the relationship between clinical, biochemical and pathological features and survival. Eur J Cancer. 1990;26:574-7.
35. Wyld L, Gutteridge E, Pinder SE, et al. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer. 2003;89:284-90.
36. Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
37. Clark GM, Sledge GW, Jr., Osborne CK, McGuire WL. Survival from first recurrence: relative importance of prognostic factors in 1,015 breast cancer patients. J Clin Oncol. 1987;5:55-61.
38. Samaan NA, Buzdar AU, Aldinger KA, et al. Estrogen receptor: a prognostic factor in breast cancer. Cancer. 1981;47:554-60.
39. O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10 Suppl 3:20-9.
40. Lee YT. Breast carcinoma: pattern of recurrence and metastasis after mastectomy. Am J Clin Oncol. 1984;7:443-9.
41. Chua TC, Saxena A, Liauw W, et al. Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer. 2011;
42. Adam R, Aloia T, Krissat J, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg. 2006;244:897-907; discussion 07-8.
43. Elias D, Maisonnette F, Druet-Cabanac M, et al. An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am J Surg. 2003;185:158-64.
44. Hoffmann K, Franz C, Hinz U, et al. Liver resection for multimodal treatment of breast cancer metastases: identification of prognostic factors. Ann Surg Oncol. 2010;17:1546-54.
45. Thelen A, Benckert C, Jonas S, et al. Liver resection for metastases from breast cancer. J Surgical Oncol. 2008;97:25-9.