Selecting Adjuvant Endocrine Therapy for Breast Cancer
This year alone, more than 215,000 women in the United States will bediagnosed with, and over 40,000 will die from, invasive breast cancer.Recently, mortality from female breast cancer has declined despite anincrease in its incidence. This decline corresponds with improved screeningfor prompt tumor detection, and advances in the treatment of earlydisease. Of these, endocrine therapy has played a prominent role. Forwomen with estrogen receptor (ER)-positive and/or progesterone receptor(PR)-positive breast cancers, endocrine therapy has proven to be amajor component of adjuvant therapy, but it is not effective in womenwhose breast cancers lack ERs and PRs. The selective estrogen-receptormodulator (SERM) tamoxifen has been well established as safe and effectivein the adjuvant care of both pre- and postmenopausal women withhormone-receptor–positive early breast cancer. For premenopausalwomen, ovarian suppression is an important option to be considered.Additionally, the aromatase inhibitors have recently demonstrated utilityin postmenopausal women. The ideal sequencing of treatment withtamoxifen and/or an aromatase inhibitor is the subject of several ongoingstudies. Factors involved in selecting an appropriate endocrine regimenhave grown considerably over the past decade. It is becoming more importantfor those caring for women with breast cancer to fully understandthe available endocrine treatment options and the prognostic and predictivefactors available to help select the most appropriate treatment. Thegoal of this article is to assist clinicians in making decisions regardingadjuvant hormonal therapy and to provide information regarding availableclinical trials. To achieve this, the therapeutic options for hormonaltherapy will be reviewed, as will prognostic and predictive factors used inmaking decisions. Finally, four cases illustrating these difficult decisionswill be discussed, with recommendations for treatment.
This year alone, more than 215,000 women in the United States will be diagnosed with, and over 40,000 will die from, invasive breast cancer. Recently, mortality from female breast cancer has declined despite an increase in its incidence. This decline corresponds with improved screening for prompt tumor detection, and advances in the treatment of early disease. Of these, endocrine therapy has played a prominent role. For women with estrogen receptor (ER)-positive and/or progesterone receptor (PR)-positive breast cancers, endocrine therapy has proven to be a major component of adjuvant therapy, but it is not effective in women whose breast cancers lack ERs and PRs. The selective estrogen-receptor modulator (SERM) tamoxifen has been well established as safe and effective in the adjuvant care of both pre- and postmenopausal women with hormone-receptor–positive early breast cancer. For premenopausal women, ovarian suppression is an important option to be considered. Additionally, the aromatase inhibitors have recently demonstrated utility in postmenopausal women. The ideal sequencing of treatment with tamoxifen and/or an aromatase inhibitor is the subject of several ongoing studies. Factors involved in selecting an appropriate endocrine regimen have grown considerably over the past decade. It is becoming more important for those caring for women with breast cancer to fully understand the available endocrine treatment options and the prognostic and predictive factors available to help select the most appropriate treatment. The goal of this article is to assist clinicians in making decisions regarding adjuvant hormonal therapy and to provide information regarding available clinical trials. To achieve this, the therapeutic options for hormonal therapy will be reviewed, as will prognostic and predictive factors used in making decisions. Finally, four cases illustrating these difficult decisions will be discussed, with recommendations for treatment.
The incidence of female breast cancer in the United States has been rising, with an estimated 215,990 new cases in 2004.[1] Despite this, there has been a decline in breast cancer mortality.[2] This decline in mortality is paralleled by an increasing use of adjuvant hormonal therapy, which is associated with an increase in survival.[3] Selecting an appropriate endocrine regimen has become more complex as choices of endocrine therapy expand. Until that time when we can identify with certainty the specific patients who benefit from adjuvant care, meaningful counseling will require not only an understanding of the risks and benefits of the various treatments, but also an appreciation of the patient's perspective. Issues regarding reproduction, body image, sexuality, and timing of side effects are part of the quality-of-life decisions facing women with breast cancer and those who would advise them.[4] Since Beatson first reported the benefit of surgical oophorectomy in the management of breast cancer over a century ago,[5] the role of endocrine therapy has evolved. Synthetic estrogen use was first reported by Haddow et al in 1944.[6] Shortly thereafter, adrenalectomy and hypophysectomy in women with metastatic breast cancer were shown to have a benefit in postmenopausal women.[7,8] Advances in our understanding of the endocrine pathways have since revealed the mechanisms by which these early surgical methods function. There are now multiple options for blocking the hormonal stimulation of tumors by estrogens, the basis of adjuvant hormonal therapy. These options include selective estrogen-receptor modulators (SERMs), aromatase inhibitors, and medical oophorectomy with luteinizing hormone-releasing hormone (LHRH) agonists.
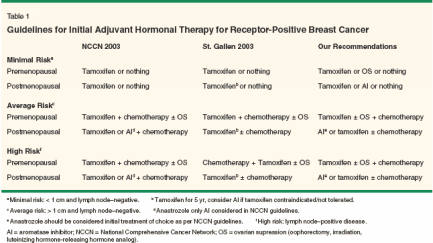
In this article, we will review the various options available and discuss their efficacy, side effects, and relevant ongoing clinical trial options. We will also review the prognostic and predictive factors that can be used to make informed decisions regarding adjuvant therapy. We will present four cases that illustrate difficulties in choosing adjuvant therapy and finally summarize our recommendations and the major consensus for adjuvant hormonal therapy (Table 1). Treatment Options Optimum use of adjuvant hormonal therapy is dependent on menopausal status. Ovarian ablation-either permanent (using surgical or radiotherapeutic ablation) or temporary (using pharmacologic agents such as LHRH agonists)-is a potential strategy for premenopausal women. The aromatase inhibitors are appropriate only for postmenopausal women and can be used in conjunction with LHRH agonists. SERMs such as tamoxifen are a viable option regardless of hormonal status. Table 2 reviews all endocrine therapies used in the treatment of breast cancer. This article will focus only on those treatments indicated for use in adjuvant therapy. Selective Estrogen-Receptor Modulators
The SERMs have varying estrogenic or antiestrogenic effects, depending on type and target tissue. The most widely studied SERM, tamoxifen, has become a treatment standard backed by several well-constructed clinical trials as well as the overview analysis.[ 3] Tamoxifen is the only SERM currently approved by the US Food and Drug Administration (FDA) for use in the adjuvant setting. Women with estrogen receptor (ER)-positive breast tumors who have completed 5 years of tamoxifen will derive a 47% reduction in annual rate of relapse and a 26% reduction in annual rates of breast cancer-related death, regardless of menopausal status.[3] Women with node-positive disease achieve greater absolute benefit from tamoxifen at 10 years (15.2% decrease in recurrence and 10.9% reduction in mortality) compared to nodenegative women (14.9% decrease in recurrence and 5.6% reduction in mortality).[3] The optimal duration of adjuvant tamoxifen therapy is probably 5 years. Adjuvant tamoxifen use for 1, 2, and 5 years has been associated with relative recurrence reductions of 21%, 29%, and 47%, respectively, and relative mortality reductions of 12%, 17%, and 26%.[3] Current data suggest no additional benefit from treatment with more than 5 years of tamoxifen,[9] and in lymph node- negative breast cancer patients, there may be a disadvantage to longer treatment periods.[10,11] Importantly, no significant decrease in cancer recurrence or improvement in survival is seen when tamoxifen is given to women with ER-negative tumors.[3] Adjuvant tamoxifen for 5 years has also been shown to reduce the risk of contralateral breast cancer by 47%.[3] While patients treated with tamoxifen have a higher proportion of ER-negative second primary breast cancers as compared with those who did not receive tamoxifen, the absolute numbers of ER-negative second primary tumors are the same and patient survival does not appear to be significantly impaired.[12] Whether tamoxifen is effective in reducing the incidence of contralateral breast cancers in women with ERnegative primary tumors remains a matter of debate. The Early Breast Cancer Trialists' Collaborative group overviews from 1998 and 2001 demonstrated a decreased rate of contralateral breast cancer when all patients, irrespective of tumor ER status, took tamoxifen for 5 years.[3,13] Other studies performed by the National Surgical Adjuvant Breast and Bowel Project (NSABP) and the Southwest Oncology Group (SWOG) showed no decrease in contralateral breast cancers with the addition of tamoxifen for women with high-risk, ER-negative, node-negative tumors.[14-16]
- Side Effects-The side-effect profile of SERMs is in great measure a function of their relative agonist and antagonist properties. Agonist effects on bone allow tamoxifen to attenuate osteoporosis in postmenopausal women. Paradoxically, it can increase calcium loss in premenopausal women.[17] In the liver, the use of tamoxifen can improve the lipid profile. Tamoxifen is associated with a two- to threefold increased risk of thrombosis, which is more pronounced for older women.[18] Although the mechanism is not well understood, reduced levels of antithrombin III and protein S have been seen in women on tamoxifen.[19] Effects on the hypothalamicgonadal axis contribute to the problematic vasomotor symptoms (hot flashes). Additionally, there is a 10% increased absolute risk of menopause over the first year of use for women over 45 years old.[20] Women younger than age 45, however, have no significant increased risk of premature menopause during this period.[20] Tamoxifen's stimulatory effect on the endometrium of postmenopausal women is associated with an increased risk of endometrial cancer and, rarely, endometrial sarcoma. For postmenopausal women, the relative risk of developing endometrial cancer after 5 years of tamoxifen is approximately 1%.[21,22] Endometrial hyperplasia, polyps, and ovarian cysts are also seen. Tamoxifen has also been associated with retinopathy, macular edema, and subcapsular cataracts.[23] Annual eye exams, screening pelvic exams, and Pap smears are recommended for women while receiving tamoxifen therapy and for 1 year upon completion of treatment.
Ovarian Ablation/Suppression
Oophorectomy has been shown to be of benefit in the adjuvant setting for premenopausal women with hormone- receptor-positive breast cancer.[ 24,25] There are now several options for ovarian suppression or ablation. Permanent ablation can be achieved surgically or with irradiation, and temporary suppression can be achieved using one of several LHRH agonists. Surgical oophorectomy is irrevers ible and, when performed laproscopically, carries minimal risk. It also provides a 90% decrease in the risk of ovarian cancer for all women, and for premenopausal women with germ-line abnormalities in BRCA1/2, oophorectomy may confer a 50% reduction in the risk of breast cancer.[26,27] It is important to note that oophorectomy, in this setting, should be discussed with a gynecologist who is familiar with the issues of ovarian cancer risk reduction to consider complete hysterectomy or just the removal of fallopian tubes and ovaries. Ovarian irradiation, on the other hand, may appeal to many by avoiding surgery. Such therapy can be accomplished with the use of either single or multiple fractions; however, on rare occasions, menses may return. The LHRH agonists triptorelin (Trelstar), goserelin (Zoladex), or leuprolide may also be utilized to achieve ovarian suppression. These agents downregulate LHRH receptors, causing a decline in leuteinizing hormone (LH) and follicle-stimulating hormone (FSH) release from the pituitary, with a subsequent drop in systemic estrogen levels. Adverse side effects are those of menopause and include headache, vasomotor symptoms, depression, emotional lability, sexual dysfunction, and vaginitis. This abrupt menopause may be poorly tolerated. Monitoring of bone mineral density for the treatment of osteoporosis is required with ovarian ablation. Bone loss occurring with ovarian suppression is likely to be reversible[28] or attenuated with bisphosphonate therapy.
- Ovarian Suppression vs Chemotherapy- Adjuvant ovarian ablation in women with hormone-receptor- positive breast cancer has been associated with a significant reduction in the annual risk of relapse (25%) and of dying of breast cancer (24%).[24] However, no significant difference has been observed when ovarian ablation is added to chemotherapy.[24,25] It may be that much of the observed benefit from adjuvant chemotherapy is derived from its ability to induce menopause in these trials, dampening the likelihood of observing a further reduction in relapse. The incidence of chemotherapy-induced amenorrhea is a function of both patient age and chemotherapy regimen, with reported rates of 40% to 70%.[29,30] Increases in both relapse-free and overall survival have been demonstrated for women who become amenorrheic after chemotherapy over those with continued menses.[31,32] Other studies have failed to demonstrate similar benefits of chemotherapyinduced amenorrhea.[33,34] Several trials have compared ovarian inhibition vs polychemotherapy and demonstrated similar efficacy in disease-free and overall survival in premenopausal woman with hormone-receptor-positive tumors.[35-37] However, these investigations did not use anthracycline- or taxane-based therapy, and thus, ovarian suppression has not been compared to current optimal chemotherapy. Quality of life may be better with ovarian suppression as compared to chemotherapy.[4] Therefore, it is important to compare ovarian suppression to current, more effective chemotherapy regimens. Given the risk of amenorrhea with chemotherapy, women hoping to maintain fertility may opt for ovarian suppression with LHRH agonists. To reduce the risk of permanent amenorrhea and provide adjuvant ovarian suppression therapy, an LHRH agonist could be started 1 month prior to initiation of chemotherapy.[ 38] Currently, this strategy should be used with caution and considered experimental. Return of menses after an LHRH agonist may not be equivalent to fertility, and the estrogen withdrawal associated with LHRH agonists may theoretically slow tumor cell growth, resulting in loss of chemosensitivity.
Aromatase Inhibitors
In postmenopausal women and in women for whom an early menopause has been induced with ovarian ablation, aromatase inhibitors may represent the most effective endocrine option. Aromatase converts testosterone to estrogen and androstenedione to estrone in adipose, muscle, breast, and breast cancer cells. By blocking this conversion, estrogen levels are decreased by more than 90%. The aromatase inhibitors have been grouped into three generations. The first generation includes aminoglutethimide (Cytadren), originally used in breast cancer management as a means of medical adrenalectomy. It has significant toxicity, causing it to be mostly of historical significance. The second-generation agents include the nonsteroidal rogletimide and fadrozole (approved for use in Japan) and the steroidal formestane (administered intramuscularly). Selective antiaromatase drugs comprise the third generation of aromatase inhibitors and are currently the aromatase inhibitors of choice. These include the steroidal, irreversible "suicide" inhibitor exemestane (Aromasin) and the nonsteroidal reversibly binding anastrozole (Arimidex), letrozole (Femara), and vorozole (R83842). Despite an increased risk of bone demineralization and frequent myalgias/ arthralgias, the aromatase inhibitors are generally well tolerated.[ 39,40] Anastrozole is associated with lower rates of thromboembolic events than tamoxifen. However, higher rates of ischemic cardiovascular events were reported with anastrozole than with tamoxifen, although this difference was not statistically different.[ 41] Lower rates of endometrial cancer and vaginal bleeding are seen with aromatase inhibitors.
- First-Line/Neoadjuvant Setting- As first-line agents in postmenopausal women with metastatic hormonereceptor- positive breast cancer, the aromatase inhibitors have demonstrated equal to superior time to progression and response rates when compared with tamoxifen,[42-44] and in the neoadjuvant setting, a decreased time to response.[45] In fact, some investigators maintain that aromatase inhibitors may be superior to chemotherapy for the neoadjuvant management of hormone-receptor-positive tumors by causing uniform, concentric tumor shrinkage with less multifocal residua and a greater likelihood of complete tumor excision at surgery.[46] Furthermore, neoadjuvant chemotherapy may be less effective in ER-positive tumors than ER-negative tumors (the inverse of which is true for neoadjuvant hormonal therapy).[47]
- Adjuvant Setting-The benefit of the aromatase inhibitors in the firstline and neoadjuvant settings has led to the question of possible superiority to tamoxifen in the adjuvant setting. Results reported by the Arimidex, Tamoxifen Alone or in Combination (ATAC) trialists' group after a median follow-up of only 47 months demonstrate that anastrozole provides a 14% reduction in relative risk of recurrence and 46% reduction in relative risk of a second primary compared to tamoxifen.[39,40] Further followup of this and other studies of adjuvant therapy should help us compare the relative risks and benefits of tamoxifen vs anastrozole, letrozole, and exemestane. Additional studies have focused on the role of adjuvant aromatase inhibitor therapy subsequent to tamoxifen; results from four of these trials have been released.[48-51] Early closure of the letrozole trial led by the National Cancer Institute of Canada (NCIC)[49] was recommended after a median follow- up of 2 years. Interim analysis demonstrated a significant increase in event-free survival for women randomized to letrozole after tamoxifen (compared to placebo). Two additional trials have recently been reported-one using anastrozole[ 50] and another, exemestane[51] begun after 2 to 3 years of tamoxifen, to complete a 5-year adjuvant treatment course, compared to tamoxifen alone for 5 years. Both of these studies confirmed an increase in diseasefree survival with transition to an aromatase inhibitor. Of note, a trial of sequential aminoglutethimide following 2 years of tamoxifen vs tamoxifen for 5 years (the predecessor to the above anastrozole study) demonstrated a mortality benefit with sequential therapy.[48] However, owing to the poor sideeffect profile of aminoglutethimide and the advent of the third-generation aromatase inhibitors, this trial failed to recruit its planned number of patients, and its results should be considered preliminary. More recently, in a presentation updating the above NCIC MA.17 letrozole trial, a significant increase in overall survival was observed for the node-positive subset of patients (personal communication, H.B. Muss, 2004).[52] The current role of aromatase inhibitors in the adjuvant setting is controversial. The four trials discussed above include over 16,000 women and although the follow-up is short, all show decreased relapse rates of breast cancer for women on aromatase inhibitors compared to tamoxifen. Not only are these data compelling, but they confirm what we already know regarding the superiority of aromatase inhibitors in the metastatic setting. It is, therefore, reasonable to consider using aromatase inhibitors for the majority of postmenopausal women except for those at lowest risk of recurrence, in whom survival data associated with tamoxifen are an important part of the risk/benefit ratio. For women currently on tamoxifen, it is reasonable to consider changing to an aromatase inhibitor, again for those at higher risk of recurrence or intolerant of tamoxifen. We suggest specific aromastase inhibitor selection be determined by the currently available trial information. That is, anastrozole as first-line adjuvant therapy, exemestane after 2 to 3 years of tamoxifen, and letrozole after 5 years of tamoxifen.
Clinical Trials The results of recent clinical trials have given us many options to consider when making choices of adjuvant hormonal therapy; however, many questions remain. These questions are the subject of four ongoing clinical trials.
- Premenopausal Women-For premenopausal women, three trials are available. For those unsure of the role of ovarian suppression, the Suppression of Ovarian Function Trial (SOFT) is comparing three strategies for adjuvant hormonal therapy: tamoxifen for 5 years, tamoxifen and ovarian suppression for 5 years, or exemestane and ovarian suppression for 5 years. Ovarian suppression may be achieved by using either the LHRH agonist triptorelin, triptorelin, surgical oophorectomy, or ovarian irradiation. Women may receive chemotherapy and may be entered and randomized up to 6 months after completion of chemotherapy, providing they are premenopausal by estradiol level. For those who are more convinced that ovarian suppression is warranted in premenopausal patients, the Tamoxifen and Exemestane Trial (TEXT) should be considered. All women in this trial will receive ovarian suppression as outlined above. Women may receive chemotherapy but ovarian suppression must be initiated at the start of chemotherapy. Women in this trial will be randomized to tamoxifen or exemestane for 5 years. For both the SOFT and TEXT trials, chemotherapy is optional and the choice of regimen is up to the discretion of the treating physician. For patients willing to be randomized to chemotherapy and convinced of the importance of ovarian ablation, the Premenopausal Endocrine Responsive Chemotherapy (PERCHE) trial may be most appropriate. Women electing to participate will be randomized to chemotherapy (of the physician's choice) or no chemotherapy and hormonal therapy with either tamoxifen or exemestane; all patients receive ovarian suppression in this trial. In all three of these trials (SOFT, TEXT, PERCHE), ovarian suppression may be permanent (oophorectomy or ovarian irradiation) or temporary (using the LHRH agonist triptorelin for 5 years).
- Postmenopausal Women-For postmenopausal women and their oncologists who may be convinced of the value of aromatase inhibitors in adjuvant treatment, enrollment in the MA.27 trial should be considered. This study, conducted by the NCIC, compares the efficacy of steroidal to nonsteroidal aromatase inhibitors (anastrozole or exemestane) for 5 years. In addition, patients are randomized to celecoxib (Celebrex) or a placebo. More information regarding eligibility and treatment can be found in Table 3. All of these trials are available through the Clinical Trials Support Unit (CTSU) of the National Cancer Institute. For more information, visit www.ctsu.org.

Prognostic and Predictive Factors "Prognostic factors" are used to predict disease outcome, whereas "predictive factors" refer to the likelihood of response to a specific treatment. The most important predictor of response to hormonal therapy is ER and PR expression. Patients with invasive breast cancer totally lacking expression of these hormone receptors will not benefit from hormonal therapy. Receptor status has often been poorly reported, and criteria for defining a receptor as "positive" are not well standardized. Some data suggest that even patients with 1% of cells staining positively for hormone receptors may derive benefit from adjuvant hormonal therapy.[53] Thus, it is important to understand the method of reporting receptor status, as many laboratories continue to report staining of less than 10% as negative. Well-validated prognostic factors for breast cancer include lymph node status, tumor stage, tumor grade, and patient age.[54] The number of lymph nodes containing tumor remains the most important prognostic factor. In addition, the extent of tumor involvement of the lymph node is important. The prognosis of micrometastasis (< 2 mm) is similar to that of uninvolved lymph nodes, and tumor deposits less than 0.2 mm are considered node-negative.[55] Additionally, the presence of lymphovascular invasion within the breast has been correlated with lymph node status and may be an independent prognostic marker.[56] The significance of lymph node status is demonstrated in the most recent National Comprehensive Cancer Network (NCCN) recommendations. These guidelines recommend adjuvant chemotherapy for anyone with even a single macroscopically positive lymph node and propose "consideration" for adjuvant therapy for those with micrometastasis.[ 57] Tumor size is another important prognostic factor, with larger tumors being more likely to recur than their smaller counterparts. Chemotherapy has been associated with a survival benefit in women who have tumors greater than 1 cm.[58] The NCCN guidelines recommend adjuvant hormonal therapy for receptor-positive tumors greater than 1 cm and consideration of hormonal therapy for tumors smaller than 1 cm.[57] Histologic grade is also of importance. Although there can be interexaminer variability, at the extremes of the grading system there is likely to be greater agreement. Patients with grade 1, node-negative tumors have been reported to have a 97% 5-year disease-free survival rate, as compared with a 78% 5-year disease-free survival for women with grade 3 nodenegative tumors.[59] Tubular or colloid histology also carries a better prognosis than ductal, lobular, or mixed tumors.[60] Tumor proliferation rates may correlate with a higher tumor grade and, therefore, greater risk of recurrence. Demographic factors such as age may also have prognostic value. Some studies suggest that older women have a poorer survival,[61] whereas other data suggest an inverse correlation between patient age and prognosis.[ 62] However, data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute show no difference in overall survival, after adjustment for stage, in patients 40 to 80 years of age.[63] Ethnic background may also be prognostic. The highest age-adjusted death rates from female breast cancer in the United States for the years 1996 to 2000 were seen in the African-American population, while the lowest were seen in women of Asian/Pacific Island descent.[1] HER2, a tyrosine kinase cell-surface receptor in the epidermal growth factor family, is overexpressed in roughly 25% of breast cancers (30%-40% of all metastatic breast cancers). It has both predictive and prognostic value. Overexpression in node-negative tumors has been associated with a poor prognosis.[ 64] Data suggest that HER2 overexpression may be an indicator of endocrine resistance, especially to tamoxifen.[65] These are provocative data that require confirmation. Currently, HER2 is not recommended for use as a predictive factor to help determine the utility of hormonal therapy. Patients can be grouped as being at either low or high risk of recurrence based on the above discussed prognostic and predictive factors. Patients with an excellent prognosis may derive little benefit from additional therapy. The therapeutic risk vs benefit ratio will vary depending on the side-effect profile of an adjuvant treatment and a patient's comorbidities. Risk Assessment Tools
Expert teams of clinicians and programmers have developed algorithms to define risk and guidelines for treatment recommendations. The two commonly used tools to define risk- Numeracy[66] and Adjuvant![67]- are available to health-care providers at the following websites: www.mayoclinic.com/calcs (Numeracy) and www.adjuvantonline.com (Adjuvant!).[ 68] Numeracy uses grouped information regarding a patient's age, nodal status, tumor size, and hormonereceptor status and provides baseline prognosis and 10-year outcome estimates based on expert panel assessment.[ 69] Adjuvant! uses the same information as well as evaluation of patient comorbidities and tumor grade incorporated with the Surveillance, Epidemiology, and End Results (SEER) database to arrive at 10-year outcome estimates.[70] A good degree of concurrence for these two methods has been demonstrated.[66] It is important to note that these programs place people in categories and that the statistics they offer refer to populations, not specific patients. The use of gene-expression analysis may prove to be a better way of defining risk of recurrence and response to therapy. This technique has been used to identify distinct patterns of gene expression in breast cancer tissue, which may allow a more accurate typing of breast cancer.[70,71] More recently, the expression of 21 specific genes was demonstrated to predict the risk of relapse in a group of 460 receptor-positive, node-negative patients taking tamoxifen.[72,73] This predictive assay is now commercially available (from Genomic Health Inc; www.genomichealth.com). While available results from this test are provocative, further confirmation in larger numbers of patients is required. Physicians wishing to use this clinical test should be careful not to overinterpret the results, especially for lymph node- positive patients or those taking aromatase inhibitors (as these patients were not included in the data set). Treatment recommendations for breast cancer based on accepted prognostic and predictive factors have been proposed by the NCCN and attendees of the 2003 St. Gallen conference.[ 57,74] Despite these general recommendations, treating oncologists are expected to appropriately integrate prognostic and predictive factors into their treatment recommendations. The four cases below illustrate the use of prognostic and predictive factors in making choices about adjuvant hormonal therapy. Case Studies
- Case 1-A.I. is an 83-year-old woman found to have a 4-cm, moderately differentiated, infiltrating ductal carcinoma with a focus of lymphovascular invasion. ERs and PRs stain positively in 80% and 90% of cells, respectively, and HER2 is overexpressed by immunohistochemistry (IHC) and amplified by flourescence in situ hybridization (FISH). There are clinically palpable lymph nodes, but the remainder of staging is negative. Ms. I. is referred to you to discuss neoadjuvant therapy. Preoperative, or neoadjuvant, therapy has been well studied utilizing both cytotoxic agents[75] and hormonal agents.[64,76] While these studies have not shown a survival benefit, neoadjuvant therapy may downstage large tumors, thereby allowing previous candidates for mastectomy to undergo breast-conserving surgery. It may also allow for a less extensive surgery and better cosmetic results. In the postmenopausal setting, the aromatase inhibitors have been demonstrated to provide superior first-line therapy over tamoxifen in metastatic disease[42,43] and are likely to be more effective neoadjuvant agents, particularly in ER-positive, HER2- overexpressing tumors.[64] A significant history of thromboembolic disease or concern for endometrial pathology would also argue for using an aromatase inhibitor, whereas a history of osteoporosis may argue against this option. With initiation of an aromatase inhibitor, bone densitometry should be evaluated at baseline and bisphosphonate management initiated as indicated. This case emphasizes a need to balance therapeutic risk with potential treatment benefits. At 83 years of age, a woman's median life expectancy is roughly 6 to 8 years.[77] This estimate can be adjusted by a patient's comorbidities, with shorter survival for women who have multiple comorbidities. In this case, we recommend initiation of an aromatase inhibitor as neoadjuvant therapy. The tumor and axillary nodes should be monitored by physical examination monthly and ultrasound as appropriate. The optimum duration of neoadjuvant hormonal therapy has not been defined; most studies have used 3 to 4 months. It would be reasonable to extend therapy to a maximum response, followed by surgery and/or definitive radiation therapy. The aromatase inhibitor should be continued for at least 5 years.
- Case 2-S.M. is a 48-year-old premenopausal woman who underwent a partial mastectomy and lymph node dissection for a 0.9-cm, well differentiated, infiltrating ductal carcinoma. ERs and PRs each stained positively in 70% of cells and HER-2 was negative. None of 16 lymph nodes were positive (T1b, pN0, M0; stage I; American Joint Committee on Cancer [AJCC] version 6). Ms. M. is referred to you while she is awaiting locoregional radiation therapy. Without any additional therapy, women like Ms. M. have an 80% to 90% 10-year relapse-free survival. Ms. M. gains greatly from treatment with tamoxifen, which would decrease her annual odds of recurrence by 47%, increase her annual odds of survival by 26%, and decrease her risk of a second primary by 47%. Ovarian suppression would also have an effect on mortality. As this woman is premenopausal, aromatase inhibitors alone would not be appropriate. She would gain additional benefit from chemotherapy; however, the short- and longterm risks (including a very low but real risk of developing acute leukemia) probably outweigh the benefits. Recent data suggesting potential equivalence of CMF chemotherapy (cyclophosphamide [Cytoxan, Neosar], methotrexate, fluorouracil [5-FU]) to ovarian ablation/suppression makes the latter an option to consider.[35] It may be argued that this 48-yearold woman may soon become postmenopausal, and as a result, she might gain little from marginally advancing this time frame. However, ovarian suppression could theoretically, permit use of an aromatase inhibitor. From the standpoint of bone health, it might be argued that younger women will have more risk of osteoporosis from an interval increase in the duration of their postmenopausal status, while someone like Ms. M. will have only a modest increase. The American Society of Clinical Oncology (ASCO) recommends that women on aromatase inhibitors hibitors should undergo annual monitoring of bone density, supplementation with calcium and vitamin D, and initiation of bisphosphonate therapy as indicated. We would encourage Ms. M. to participate in either the TEXT or SOFT trial (see Table 3). Off study, we would recommend tamoxifen.
- Case 3-M.O. is a 39-year-old woman who underwent a partial mastectomy and axillary dissection for a 1.9-cm, poorly differentiated, infiltrating ductal carcinoma. ERs and PRs each stained positively in 90% of cells. Of 17 axillary lymph nodes, 2 were positive (T1c, pN1a, M0; stage IIA; AJCC version 6). Ms. O. is referred to you to discuss adjuvant therapy and the sequence of that therapy. Compared with case 2, there are several aspects of Ms. O.'s presentation (such as lymph node status, tumor size, histologic grade, and patient age) that place her in a higher risk category. Patients like Ms. O. can be expected to have a 10-year relapse rate of around 50%. Furthermore, 30% to 40% of patients like Ms. O. would be expected to die from their cancer during this interval. For this reason, more aggressive adjuvant care would be recommended. Tamoxifen alone would provide a 44% and 25% relative risk reduction in relapse and mortality, respectively. The Adjuvant! decision aid would predict roughly 18 fewer relapses and 7 fewer deaths over 10 years for every 100 women treated with tamoxifen.[68] Chemotherapy alone would also significantly reduce the risk of recurrence and death. Depending on the regimen used, she might have as much as a 46% reduction in risk of relapse and 36% reduction in mortality (with a dosedense regimen for example). This would translate to roughly 19 fewer relapses and 9 fewer deaths in a 10-year period for every 100 women treated.[68] Combined use of chemotherapy and hormonal therapy, while not additive, should further decrease risk by preventing approximately 32 relapses and nearly 15 deaths from occurring over 10 years for every 100 women treated.[68] Treatment guidelines for this group of women with node-positive and receptor-positive breast cancer suggest combined chemotherapy and hormonal therapy.[57,75] The option of ovarian suppression/ ablation should be seriously considered in this individual. Although CMF and ovarian suppression/ablation may be equivalent, it is not known if overall survival is equivalent to newer, more effective chemotherapies including the anthracyclines and taxanes. Temporary ovarian suppression may preserve fertility for women who have not completed childbearing. While it is not recommended that women conceive during or soon after therapy (first 2 to 5 years), these recommendations are made to avoid negative effects of therapy on pregnancy and allow a significant time for the greatest risk of relapse to pass. Women with strong family histories of breast and ovarian cancer may harbor germ-line mutations in BRCA1/2, and prophylactic oophorectomy may be considered not only to decrease the risk of recurrence but also to decrease the risk of a second primary breast cancer and ovarian cancer. For this individual with two positive nodes, we would recommend both chemotherapy and hormonal therapy. The choice of hormonal therapy is difficult to make. Current information regarding the utility of ovarian suppression in addition to tamoxifen would support using this strategy.[36] To potentially preserve fertility, an LHRH agonist could be initiated prior to chemotherapy, although as previously mentioned, there is no guarantee of fertility, and hormonal therapy during chemotherapy could have a negative impact. This patient may be most appropriately considered for the TEXT trial, as all women enrolled in this study receive ovarian suppression initiated prior to chemotherapy. Consideration of the PERCHE trial would mean a randomization to chemotherapy or not, and she would only be eligible for the SOFT trial if she had menses after chemotherapy.
- Case 4-E.B. is a 63-year-old woman who underwent a mastectomy with lymph node dissection, revealing a 2.8-cm, moderately differentiated, infiltrating ductal carcinoma. The tumor was ER-positive in 60% of cells and PR negative. HER2 was 3+ overexpressed (positive) by IHC. Of 11 axillary lymph nodes, 3 were positive for tumor (T2, pN1a, M0; stage IIB; AJCC version 6). Ms. B.'s past medical history is significant for a recent transient ischemic attack. She is referred to you to discuss adjuvant hormonal therapy. Ms. B. and Ms. O. (case 3) have similar-stage disease, and they share a similar prognosis should no additional therapy be provided. Without adjuvant treatment, we would expect roughly half of the women with Ms. B.'s history to have their cancers relapse, and one-third die from their cancer within 10 years of diagnosis. For reasons that may be linked to the hormonal effects of chemotherapy, the incremental benefit Ms. B. may be expected to receive from chemotherapy is less than that of her premenopausal counterpart. Using dosedense chemotherapy, a potential 30% and 26% relative risk reduction in relapse and mortality, respectively, would translate to roughly 11 (vs 19) fewer relapses and 7 (vs 9) fewer deaths in a 10-year period for every 100 women treated.[68] The choice of hormonal therapy (tamoxifen vs aromatase inhibitor), duration of hormonal therapy, and potential for sequencing of hormonal therapy remain complicated decisions for patients and providers. In postmenopausal patients, adjuvant treatment with tamoxifen is estimated to provide roughly a 40% relative reduction in risk of relapse, while use of anastrozole provides roughly a 52% reduction.[39] However, no long-term data demonstrate a mortality benefit. Over 10 years, for every 100 women treated, this would translate into roughly 21 fewer relapses with anastrozole use, 15 fewer relapses with tamoxifen use, and 8 fewer deaths due to cancer using either agent.[68] There are three factors in this case that may provide additional weight in favor of using an aromatase inhibitor, however. First, Ms. B.'s recent transient ischemic attack would favor avoiding the increased risk of thromboembolic disease seen in postmenopausal women taking tamoxifen. Second, the fact that her tumor is ERpositive/ PR-negative favors the use of an aromatase inhibitor over tamoxifen.[ 63] Third, HER2 overexpression may suggest decreased tamoxifen sensitivity and an increased aromatase inhibitor sensitivity.[65] For Ms. B., we would recommend hormonal therapy with an aromatase inhibitor and consideration of chemotherapy. We would encourage participation in the NCIC MA.27 trial, as the optimum aromatase inhibitor is unknown (Table 3).
- Summary-These four cases illustrate the differing approaches to hormonal therapy based on the patient's risk of both breast cancer recurrence and dying. Table 1 highlights our recommendations compared to those proposed by the NCCN and at the 2003 St. Gallen conference.[57,74] For premenopausal women at the lowest risk of recurrence, we would recommend either no further therapy, tamoxifen for 5 years, or ovarian suppression. For women at average and high risk, we would consider tamoxifen for 5 years with or without ovarian suppression. Use of ovarian suppression and an aromatase inhibitor should only be done on a clinical trial. At this time, there is not enough evidence to suggest that any of the third-generation aromatase inhibitors is better than another. Premenopausal women in this category should be offered chemotherapy in addition to hormonal therapy. For postmenopausal women, we would recommend tamoxifen or an aromatase inhibitor for 5 years or no adjuvant hormonal therapy. For postmenopausal women at average or high risk of recurrence, chemotherapy should be considered in addition to hormonal therapy. Additionally, we do not recommend the use of tamoxifen in women with ER/PR-negative tumors. Conclusions For over 100 years, we have known the importance of endocrine therapy in the treatment of breast cancer. We currently have a wide array of potential endocrine treatments for every stage of cancer care. From preventive treatment for patients at high risk of developing breast cancer to therapy for hormone-responsive metastatic disease, endocrine therapy provides several avenues for effective treatment. In the hope that ongoing investigations will continue to advance our knowledge of effective treatment, we encourage women and their health-care providers to seriously consider clinical trials in therapy choices.
Disclosures:
Dr. Muss has ownership interest in Amgen and Enzon, has received research grants from Aventix, Pfizer, Celgene, Coley Pharmacia, Immunex, Schering, Merck, AstraZeneca, Imclone, Ligand, Lilly, Genentech, Bristol-Myers Squibb, Ortho-Biotech, Novartis, Genetics Institute, and Tibotech; fellowship support from Ortho, Amgen, and Aventis; honoraria from Network Oncology Communication, Neil Love Communications, and Medidigm; and is on the board of directors or advisory committees of the Amercian Society of Clinical Oncology.
References:
1. Jemal A, Tiwari RC, Murray T, et al: Cancer statistics, 2004. CA Cancer J Clin 54:8-29, 2004.
2. Peto R, Boreham J, Clarke M, et al: UK and USA breast cancer deaths down 25 percent at ages 20-69 years. Lancet 355:1822, 2000.
3. Early Breast Cancer Trialists’ Collaborative Group: Tamoxifen for early breast cancer: An overview of the randomized trials. Lancet 351:1451-1467, 1998.
4. de Haes H, Olschewski M, Kaufmann M: Quality of life in goserelin-treated versus cyclophosphamide + methotrexate + fluorouraciltreated premenopausal and perimenopausal patients with node positive, early breast cancer: The Zoladex Early Breast Cancer Research Association Trialists Group. J Clin Oncol 21:4510-4516, 2003.
5. Beatson GT: On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment with illustrative cases. Lancet 2:104-107, 1896.
6. Haddow A, Watkinson JM, Patterson E, et al: Influence of synthetic estrogens upon advanced malignant disease. Br Med J 2:393- 398, 1944.
7. Huggins C, Bergenstal DM: Inhibition of human mammary and prostatic cancer by adrenalectomy. Cancer Res 12:134-141, 1952.
8. Robin PE, Powell DJ, Waterhouse JA, et al: Transphenoidal hypophysectomy in disseminated carcinoma of the breast. Br J Surg 62:85-91, 1975.
9. Tormey DC, Gray R, Falkson HC: Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph nodepositive breast cancer. Eastern Cooperative Oncology Group. J Natl Cancer Inst 88:1828- 1833, 1996.
10. Fisher B, Dignam J, Bryant J, et al: Five vs more than five years of tamoxifen for lymph node-negative breast cancer: Updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst 93:684-690, 2001.
11. Stewart HJ, Forrest APM, Everington D, et al: Randomized comparison of 5 years of adjuvant tamoxifen with continuous therapy for operable breast cancer. Br J Cancer 74:297- 299, 1996.
12. Kass R, Peterse JL, Hart AAM, et al: The influence of tamoxifen treatment on the oestrogen receptor in metachronous contralateral breast cancer. Br J Cancer 88:707-710, 2003.
13. Early Breast Cancer Trialists’ Collaborative Group: Tamoxifen for early breast cancer. Cochrane Database Syst Rev 2001:CD000486.
14. Fisher B, Anderson S, Tan-Chiu E, et al: Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor negative breast cancer. Findings from National Breast and Bowel Project B-23. J Clin Oncol 19:931- 942, 2001.
15. Swain SM: Tamoxifen for patients with estrogen receptor negative breast cancer. J Clin Oncol 19:93s-97s, 2001.
16. Hutchins L, Green S, Ravdin P, et al: CMF versus CAF+/- tamoxifen in high-risk node-negative breast cancer patients and a natural history follow-up study in low-risk nodenegative patients: Update of tamoxifen results (abstract). Breast Can Res Treat 57:25, 1999.
17. Powles TJ, Hickish TF, Kanis JA, et al: Tamoxifen preserves bone mineral density in premenopausal women (abstract). Proc Am Soc Clin Oncol 14:165, 1995.
18. Fisher B, Costantino JP, Wickerham DL, et al: Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project p-1 study. J Natl Cancer Inst 90:1371-1388, 1998.
19. Cushman M, Constantino JP, Bovill EG, et al: Effect of tamoxifen on venous thrombosis risk factors in women without cancer: The breast cancer prevention trial. Br J Hematol 120:109-116, 2003.
20. Goodwin PJ, Ennis M, Pritchard KI, et al: Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 17:2365-2370, 1999.
21. Bernstein L, Deapen D, Cerhan JR, et al: Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst 91:1654-1662, 1999.
22. Saga Y, Ohwada M, Kohno T, et al: High grade endometrial stromal sarcoma after treatment with tamoxifen in a patient treated for breast cancer. Int J Gynecol Cancer 13:690- 692, 2003.
23. Kaiser-Kupfer MI, Lippman ME: Tamoxifen retinopathy. Cancer Treat Rep 62:315-320, 1978.
24. Early Breast Cancer Trialists’ Collaborative Group: Ovarian ablation in early breast cancer: overview of randomized trials. Lancet 348:1189-1196, 1996.
25. Early Breast Cancer Trialists’ Collaborative Group: Ovarian ablation for early breast cancer. Cochrane Database Syst Rev 2000:CD000485.
26. Kauff ND, Satagopan JM, Robson ME, et al: Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 346:1609-1615, 2002.
27. Rebbeck TR, Lynch HT, Neuhausen SL, et al: The prevention and observation of surgical end points study group: Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 346:1616-1622, 2002.
28. Paoletti AM, Serra GG, Cagnacci A, et al: Spontaneous reversibility of bone loss induced by gonadotropin-releasing hormone analog treatment. Fertil Steril 65:707-710, 1996.
29. Bines J, Oleske DM, Cobleigh MA: Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol 14:1718-1729, 1996.
30. Stone ER, Slack RS, Novielli A, et al: Rate of chemotherapy related amenorrhea (CRA) associated with adjuvant Adriamycin and Cytoxan followed by Taxol (AC+T) in early stage breast cancer (abstract). Breast Cancer Res Treat 64:61, 2000.
31. Del Mastro L ,Venturini M, Sertoli MR, et al: Amenorrhea induced by adjuvant chemotherapy in early breast cancer patients: Prognostic role and clinical applications. Breast Cancer Res Treat 43:183-190, 1997.
32. Pagani O, O’Neill A, Castiglione M, et al: Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer 34:632-640, 1998.
33. Levine MN, Bramwell VH, Pritchard KI, et al: Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer, National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 16:2651-2658, 1998.
34. Henderson IC, Berry DA, Demetri GD, et al: Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976-983, 2003.
35. Jonat W, Kaufmann M, Sauerbrei W, et al: Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: The Zoladex Early Breast Cancer Research Association Study. J Clin Oncol 20:4628-4635, 2002.
36. Jakesz R, Hausaninger H, Kubista E: Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: Evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer-Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol 20:4621-4627, 2002.
37. International Breast Cancer Study Group (IBCSG): Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node negative breast cancer: A randomized trial. J Natl Cancer Inst 95:1833-1846, 2003.
38. Fox KR, Scialla J, More H: Preventing chemotherapy-related amenorrhea using leuprolide during adjuvant chemotherapy for early-stage breast cancer (abstract 50). Proc Am Soc Clin Oncol 22:13, 2003.
39. The ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists’ Group: Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet 359:2131-2127, 2002.
40. The ATAC Trialists Group: The ATAC trial in post-menopausal women with early breast cancer-updated efficacy results based on a median follow-up of 47 months (abstract). Breast Cancer Res Treat 82, 2003.
41. Baum M, Buzdar AU, Cuzick J, et al: Anastrozole alone or in combination with tamoxifen vs tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: Results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial. Efficacy and safety update analysis. Cancer 98:1802-1810, 2003.
42. Mouridsen H, Gershanovich M, Sun Y, et al: Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: Results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 19:2596-2606, 2001.
43. Nabholtz JM, Buzdar A, Pollak M, et al: Anastrozole is superior to tamoxifen as firstline therapy for advanced breast cancer in postmenopausal women: Results of a North American multicenter randomized trial. J Clin Oncol 18:3758-3767, 2000.
44. Bonneterre J, Thurlimann B, Robertson JFR, et al: Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: Results of the tamoxifen or Arimidex randomized group efficacy and tolerability study. J Clin Oncol 18:3748-3757, 2000.
45. Eirmann W, Paepke S, Appfelstaedt J, et al: Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 12:1527-1532, 2001.
46. Dixon JM, Anderson TJ, Miller WR: Neoadjuvant endocrine therapy of breast cancer: A surgical perspective. Eur J Cancer 38:2214-2221, 2002.
47. Buzdar AU, Valero V, Theriault RL, et al: Pathologic complete response to chemotherapy is related to hormone receptor status. Breast Cancer Res Treat 82:S69, 2003.
48. Boccardo F, Rubagotti A, Amoroso D, et al: Sequential tamoxifen and aminoglutethimide versus tamoxifen alone in the adjuvant treatment of postmenopausal breast cancer patients: Results of an Italian cooperative study. J Clin Oncol 19:4209-4215, 2001.
49. Goss PE, Ingle JN, Martino S, et al: A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793-1802, 2003.
50. Boccardo F, Rubagotti A, Amoroso D, et al: Anastrozole appears to be superior to tamoxifen in women already receiving adjuvant tamoxifen treatment. Breast Cancer Res Treat 82:S6-S7, 2003.
51. Coombes RC, Hall E, Gibson LJ, et al: A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. 350:1081-1092, 2004.
52. Goss PE, Ingle JN, Martino S, et al: Updated analysis of the NCIC CTG MA.17 randomized placebo (P) controlled trial of letrozole (L) after five years of tamoxifen in postmenopausal women with early stage breast cancer, J Clin Oncol (2004 ASCO Annual Meeting Proceedings, Post-Meeting Edition) 22:847, 2004.
53. Harvey JM, Clark GM, Osborne K, et al: Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474-1481, 1999.
54. Esteva FJ, Hortobagyi GN: Breast cancer management: Adjuvant systemic therapy for primary breast cancer. Surg Clin North Am 79:1075-1090, 1999.
55. Singletary SE, Allred C, Ashley P, et al: Revision of the American Joint Committee on Cancer Staging System for Breast Cancer. J Clin Oncol 20:3628-3636, 2002.
56. Woo CS, Silberman H, Nakamura SK, et al: Lymph node status combined with lymphovascular invasion creates a more powerful tool for predicting outcome in patients with invasive breast cancer. Am J Surg 184:337- 340, 2002.
57. The NCCN: Breast cancer clinical practice guidelines in oncology. JNCCN 1:148-188, 2003.
58. Aapro MS: Adjuvant therapy of primary breast cancer: A review of key findings from the 7th international conference, St Gallen, February 2001. Oncologist 6:376-385, 2001.
59. Lundin J, Lundin M, Holli K, et al: Omission of histologic grading from clinical decision making may result in overuse of adjuvant therapies in breast cancer: Results from a nationwide study. J Clin Oncol 19:28-36, 2001.
60. Rosen PP, Groshen S, Saigo PE: Pathological prognostic factors in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma: A study of 644 patients with median follow-up of 18 years. J Clin Oncol 7:1239-1251, 1989.
61. Adami HO, Malker B, Holmbert L, et al: The relation between survival and age at diagnosis in breast cancer. N Engl J Med 315:559-563, 1996.
62. Bernstein JL, Lapinski R, Lynch C, et al: Factors influencing mortality among young women with second primary breast carcinoma. Cancer 95:2051-2058, 2002.
63. Yuneik R, Ries LG, Yates JW: Breast cancer in aging women: A population-based study of contrasts in stage, surgery, and survival. Cancer 63:976-981, 1989.
64. Andrulis IL, Bull SB, Blackstein ME, et al: neu/erbB-2 amplification identifies a poor prognosis group of women with node negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol 16:1340-1349, 1998.
65. Ellis MJ, Coop A, Singh B, et al: Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1 and/or ErbB-2-postive, estrogen receptor-positive primary breast cancer: Evidence from a phase III randomized trial. J Clin Oncol 19:3808-3816, 2001.
66. Loprinzi CL, Ravdin RM: Decision making for patients with resectable breast cancer: Individualized decisions for and by patients and their physicians. JNCCN 1:189-196, 2003.
67. Ravdin PM, Siminoff LA, Davis GJ, et al: Computer program to assist in making decision about adjuvant therapy for women with early breast cancer. J Clin Oncol 19:980-991, 2001.
68. Adjuvant! Available online at www.adjuvantonline.com. Accessed November 2, 2004.
69. Loprinzi CL, Thomé SD: Understanding the utility of adjuvant systemic therapy for primary breast cancer. J Clin Oncol 19:3795- 3797, 2001.
70. Perou CM, Sorlie T, Eisen MB, et al: Molecular portraits of human breast tumors. Nature 406:747-752, 2000.
71. Hedenfalk I, Duggan D, Chen Y, et al: Gene-expression profiles in hereditary breast cancer. N Engl J Med 344:539-548, 2001.
72. Paik S, Shak S, Tang G, et al: Multigene RT-PCR assay for prediction recurrence in node negative breast cancer patients- NSABP studies B-20 and B-14 (abstract). Breast Cancer Res Treat 82:16, 2003.
73. Esteva FJ, Sahin AA, Coombes K, et al: Multi-gene RT-PCR assay for predicting recurrence in node negative breast cancer patients- M. D. Anderson Clinical Validation Study (abstract 17). Breast Cancer Res Treat 82:S11, 2003.
74. Senn HJ, Thurlimann B, Goldhirsch A, et al: Comments of the St. Gallen Consensus 2003 on the Primary Therapy of Early Breast Cancer. The Breast 12:569-582, 2003.
75. NSABP: The effect on primary tumor response of adding sequential Taxotere to Adriamycin and cyclophosphamide: Preliminary results from NSABP Protocol B-27 (abstract). Breast Cancer Res Treat 69:210, 2001.
76. Dixon JM, Renshaw L, Bellamy C: The effects of neoadjuvant anastrozole (Arimidex) on tumor volume in postmenopausal women with breast cancer: A randomized, double blind, single center study. Clin Cancer Res 6:2229- 2235, 2000.
77. Walter LC, Covinsky KE: Cancer screening in elderly patients: A framework for individualized decision making. JAMA 285:2750- 2756, 2001. Suggested Readings
Aapro MS: Adjuvant therapy of primary breast cancer: A review of key findings from the 7th International Conference, St Gallen, February 2001. Oncologist 6:376-385, 2001. Campos SM, Winer EP: Hormonal therapy in postmenopausal women with breast cancer. Oncology 64:289-299, 2003.
Davidson NE: Controversies in adjuvant therapy for endocrine therapy-responsive breast cancer, in Perry MC (ed): American Society of Clinical Oncology 2002 Educational Book, pp 156-160. Baltimore, Lippincott Williams & Wilkins, 2002.
Early Breast Cancer Trialists' Collaborative Group: Tamoxifen for early breast cancer: An overview of the randomized trials. Lancet 351:1451-1467, 1998.
Goss PE, Strausser K: Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol 19:881-894, 2001.
Hortobagyi GN: Treatment of breast cancer. N Engl J Med 359:974-984, 1998 Muss HB: Role of adjuvant endocrine therapy in early-stage breast cancer. Semin Oncol 28:313-321, 2001.
National Comprehensive Cancer Network: Breast cancer clinical practice guidelines in oncology. JNCCN 1:148-188, 2003.
Pritchard KI: Controversies in adjuvant systemic therapy: Predictive markers in the selection of optimal systemic therapy, in Perry MC (ed): American Society of Clinical Oncology 2002 Educational Book, pp 161-173. Baltimore, Lippincott Williams & Wilkins, 2002.
Shapiro CL, Recht A: Side effects of adjuvant treatment of breast cancer. N Engl J Med 344:1997-2008.