State-of-the-Art Treatment for Advanced NonSMQ-8211-SMQSmall-Cell Lung Cancer
Patients with locally advanced or metastatic nonSMQ-8211-SMQsmall-cell lungcancer (stage III and IV) who are not candidates for surgery and exhibitgood performance status are typically treated with concurrent radiationand platinum-based chemotherapy for disease palliation. Platinum-based chemotherapies, used alone or with radiation therapy, offera small but significant survival benefit compared with supportivecare. The incorporation of first-line agents such as gemcitabine(Gemzar), vinorelbine (Navelbine), and paclitaxel, as well as secondlineagents such as docetaxel (Taxotere), in doublet and triplet combinationshas had a further significant therapeutic impact. Randomizedtrials have shown that cisplatin-based therapy in combination with newagents results in improved 1- and 2-year survival rates in patients withadequate performance status. The 1-year survival benefit has significantlyimproved, with greater symptom relief and improved quality oflife in these patients. Thus, delaying disease progression with combinationchemotherapy appears both beneficial and cost-effective in patientswith advanced nonSMQ-8211-SMQsmall-cell lung cancer. Newer approachesSMQ-8212-SMQincluding targeting critical signaling pathways, such as tyrosine kinasereceptors, angiogenesis, and downstream signal transductionmechanismsSMQ-8212-SMQmay provide novel agents with an improved toxicity profileand the potential for better disease management.
ABSTRACT: Patients with locally advanced or metastatic nonSMQ-8211-SMQsmall-cell lungcancer (stage III and IV) who are not candidates for surgery and exhibitgood performance status are typically treated with concurrent radiationand platinum-based chemotherapy for disease palliation. Platinum-based chemotherapies, used alone or with radiation therapy, offera small but significant survival benefit compared with supportivecare. The incorporation of first-line agents such as gemcitabine(Gemzar), vinorelbine (Navelbine), and paclitaxel, as well as secondlineagents such as docetaxel (Taxotere), in doublet and triplet combinationshas had a further significant therapeutic impact. Randomizedtrials have shown that cisplatin-based therapy in combination with newagents results in improved 1- and 2-year survival rates in patients withadequate performance status. The 1-year survival benefit has significantlyimproved, with greater symptom relief and improved quality oflife in these patients. Thus, delaying disease progression with combinationchemotherapy appears both beneficial and cost-effective in patientswith advanced nonSMQ-8211-SMQsmall-cell lung cancer. Newer approachesSMQ-8212-SMQincluding targeting critical signaling pathways, such as tyrosine kinasereceptors, angiogenesis, and downstream signal transductionmechanismsSMQ-8212-SMQmay provide novel agents with an improved toxicity profileand the potential for better disease management.Lung cancer is the second mostcommon cancer and the leadingcause of cancer death inboth men and women in the UnitedStates.[1] It is responsible for moredeaths than prostate, colon, and breastcancer combined, with an estimated157,200 deaths from lung cancer in2003.[1] The 5-year survival rate forpatients diagnosed with lung cancerin 2003 is projected to be 15%, reflectingonly a 2% increase over thelast 4 years.[1,2] Of the estimated172,000 cases of lung cancer in 2003,non-small-cell lung cancer will accountfor 80% to 85%.[3] Unfortunately,due to the inherent resistanceof non-small-cell lung cancer to chemotherapyand radiation therapy, coupledwith a limited understanding ofthe molecular events involved in thedevelopment and progression of lungcancer, it appears that this disease willbe one of the most challenging to overcomein the 21st century.Non-small-cell lung cancer is oneof the most aggressive types of cancers,with 30% to 40% of patients presentingwith distant metastases. Leftuntreated, the median survival time fora patient with metastatic disease isapproximately 4 to 5 months. In general,when surgery is not considered,patients with locally advanced ormetastatic non-smal--cell lung cancer(stage III and IV) and good performancestatus are treated with concurrentradiation and platinum-based chemotherapyfor palliation of symptomsfrom the primary tumor.[4,5] The timebetween diagnosis and treatment appearsto be a critical prognostic factorfor median survival. The AmericanSociety of Clinical Oncology Guidelinessupport treatment as soon as adiagnosis is made.[5] Findings suggestthat appropriate and early delivery ofchemotherapy improves patientsurvival.Studies that support these findingswill be discussed in the following sections,including those that support theuse of combination chemotherapy toprovide symptom relief and enhancesurvival time. In addition, selectednovel agents are reviewed, especiallythose targeted to pathways critical tooncogenesis.
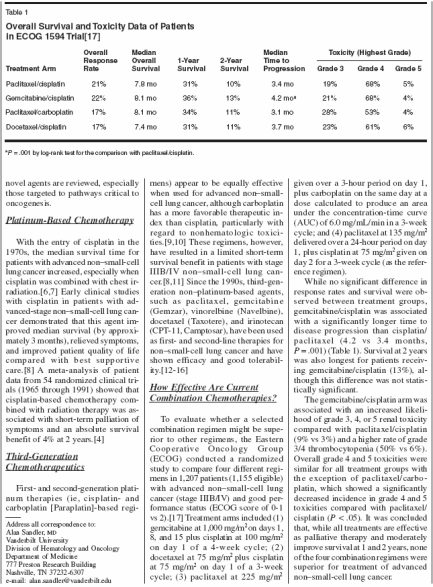
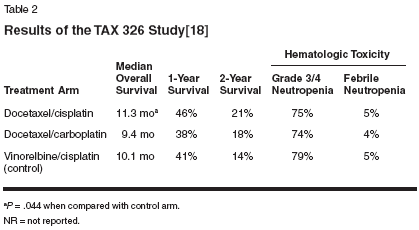
Platinum-Based ChemotherapyWith the entry of cisplatin in the1970s, the median survival time forpatients with advanced non-small-celllung cancer increased, especially whencisplatin was combined with chest irradiation.[6,7] Early clinical studieswith cisplatin in patients with advanced-stage non-small-cell lung cancerdemonstrated that this agent improvedmedian survival (by approximately3 months), relieved symptoms,and improved patient quality of lifecompared with best supportivecare.[8] A meta-analysis of patientdata from 54 randomized clinical trials(1965 through 1991) showed thatcisplatin-based chemotherapy combinedwith radiation therapy was associatedwith short-term palliation ofsymptoms and an absolute survivalbenefit of 4% at 2 years.[4]Third-GenerationChemotherapeuticsFirst-and second-generation platinumtherapies (ie, cisplatin-andcarboplatin [Paraplatin]-based regi-mens) appear to be equally effectivewhen used for advanced non-smallcelllung cancer, although carboplatinhas a more favorable therapeutic indexthan cisplatin, particularly withregard to nonhematologic toxicities.[9,10] These regimens, however,have resulted in a limited short-termsurvival benefit in patients with stageIIIB/IV non-small-cell lung cancer.[8,11] Since the 1990s, third-generationnon-platinum-based agents,such as paclitaxel, gemcitabine(Gemzar), vinorelbine (Navelbine),docetaxel (Taxotere), and irinotecan(CPT-11, Camptosar), have been usedas first- and second-line therapies fornon-small-cell lung cancer and haveshown efficacy and good tolerability.[12-16]How Effective Are CurrentCombination Chemotherapies?To evaluate whether a selectedcombination regimen might be superiorto other regimens, the EasternCooperative Oncology Group(ECOG) conducted a randomizedstudy to compare four different regimensin 1,207 patients (1,155 eligible)with advanced non-small-cell lungcancer (stage IIIB/IV) and good performancestatus (ECOG score of 0-1vs 2).[17] Treatment arms included (1)gemcitabine at 1,000 mg/m2 on days 1,8, and 15 plus cisplatin at 100 mg/m2on day 1 of a 4-week cycle; (2)docetaxel at 75 mg/m2 plus cisplatinat 75 mg/m2 on day 1 of a 3-weekcycle; (3) paclitaxel at 225 mg/m2given over a 3-hour period on day 1,plus carboplatin on the same day at adose calculated to produce an areaunder the concentration-time curve(AUC) of 6.0 mg/mL/min in a 3-weekcycle; and (4) paclitaxel at 135 mg/m2delivered over a 24-hour period on day1, plus cisplatin at 75 mg/m2 given onday 2 for a 3-week cycle (as the referenceregimen).While no significant difference inresponse rates and survival were observedbetween treatment groups,gemcitabine/cisplatin was associatedwith a significantly longer time todisease progression than cisplatin/paclitaxel (4.2 vs 3.4 months,P = .001) (Table 1). Survival at 2 yearswas also longest for patients receivinggemcitabine/cisplatin (13%), althoughthis difference was not statisticallysignificant.The gemcitabine/cisplatin arm wasassociated with an increased likelihoodof grade 3, 4, or 5 renal toxicitycompared with paclitaxel/cisplatin(9% vs 3%) and a higher rate of grade3/4 thrombocytopenia (50% vs 6%).Overall grade 4 and 5 toxicities weresimilar for all treatment groups withthe exception of paclitaxel/carboplatin,which showed a significantlydecreased incidence in grade 4 and 5toxicities compared with paclitaxel/cisplatin (P < .05). It was concludedthat, while all treatments are effectiveas palliative therapy and moderatelyimprove survival at 1 and 2 years, noneof the four combination regimens weresuperior for treatment of advancednon-small-cell lung cancer.A recent multicenter phase IIIstudy, TAX 326, was designed to comparethe safety and efficacy of platinumcombinations in advanced non-small-cell lung cancer using identicaldoses of a third-generation chemotherapeuticagent (docetaxel) incombination with comparable dosesof either cisplatin or carboplatin(Table 2).[10,18] TAX 326 was aninternational, multicenter, randomizedtrial that included 1,220 patientswith advanced non-small-cell lungcancer. Patients were randomized toreceive one of three regimens: (1)docetaxel at 75 mg/m2 plus cisplatinat 75 mg/m2 every 3 weeks; (2)docetaxel at 75 mg/m2 plus carboplatinat AUC 6 every 3 weeks; or (3)vinorelbine at 25 mg/m2 days 1, 8, 15,and 22 plus cisplatin at 100 mg/m2 day1 every 4 weeks (control arm).Median survival was 11.3 monthsin arm 1, 9.4 months in arm 2, and 10.1months in the control arm. The 1-yearsurvival rates were 46% in arm 1, 38%in arm 2, and 41% in the control arm.Arm 1 was equivalent to and nearlysuperior to arm 2 with respect to survival.The efficacy of arm 2 was notinferior to the control arm. Hematologictoxicity was similar across treatmentarms. Patients treated withvinorelbine/cisplatin had the highestincidence of nausea and vomiting.Over 900 patients completed qualityof-life evaluations. Improvements inquality of life and clinical benefit, asmeasured by reduction in pain, improvementin performance status, andlack of major weight loss, were seenon both docetaxel arms. TAX 326, thelargest trial yet conducted in advancednon-small-cell lung cancer, has demonstratedthat the combination ofdocetaxel/cisplatin is at least equivalentto the combination of vinorelbineand cisplatin, and in this study wasshown to be superior in terms ofsurvival.In a phase III study by the SwedishLung Cancer Study Group,[19]gemcitabine in combination withcarboplatin for the treatment of advanceddisease was found to significantlyincrease objective response rate(30% vs 12%) and median time toprogression (6 vs 4 months, P = .001)compared to treatment with gem-gemcitabinealone. Median survival forboth groups was 9 months. In a studyconducted by the Cancer and LeukemiaGroup B, paclitaxel in combinationwith carboplatin was associatedwith an improvement in response ratecompared with paclitaxel alone (30%vs 16%, P < .001), with a median survivalof 8.5 months for the combinationvs 6.5 months for paclitaxel alone(P = .023).Another study by Georgoulias andcolleagues[20] compared the combinationof docetaxel/cisplatin todocetaxel alone. The regimen resultedin a significantly higher objective responserate, but this did not translateinto improved overall survival. Thus,it is logical to determine which doubletcombinations are best toleratedand offer the greatest benefit for patientswith advanced non-small-celllung cancer. This was difficult, however,as no single comparative studyhad been statistically powered to addresssuch an issue.In addition to the aforementionedstudies, numerous other randomizedtrials have shown that there are severalthird-generation platinum-basedcombinations that may be consideredin patients with metastatic non-smallcelllung cancer.Triple CombinationChemotherapy RegimensTriple combination chemotherapyhas recently been evaluated to determineif this would further improvepatient outcomes relative to doubletregimens. The Spanish Lung CancerGroup conducted a phase III trial comparinga cisplatin-based triple combinationregimen, a nonplatinum sequentialdoublet regimen, and acisplatin-based regimen (as the referencearm) in 562 patients with advancednon-small-cell lung cancer.[21] Treatmentregimens consisted of arm A:cisplatin at 100 mg/m2 on day 1 plusgemcitabine at 1,250 mg/m2 on days 1and 8; arm B: cisplatin at 100 mg/m2on day 1, gemcitabine at 1,000 mg/m2on days 1 and 8, and vinorelbine at25 mg/m2 on days 1 and 8, repeatedevery 3 weeks; and arm C: gemcitabineat 1,000 mg/m2 plus vinorelbineat 30 mg/m2 on days 1 and 8 forthree cycles, followed by ifosfamide(Ifex) at 3 g/m2 on day 1 plusvinorelbine at 30 mg/m2 on days 1 and8. Eligibility criteria included measurablestage IV (brain metastases eligibleif asymptomatic) or stage IIIB (malignantpleural effusion) non-small-celllung cancer and a performance statusof 0 to 2.Overall response rates among 410eligible patients were best in arm A(41%) and arm B (40%) comparedwith arm C (24%). Median survivalwas similar for the three treatmentgroups. Toxicities observed in arms A,B, and C included grades 3/4 neutropeniain 26.3%, 30.1%, and 18.5%;neutropenic fever in 6.3%, 22.4%, and7.4%; and grades 3/4 thrombocytopeniain 18.2%, 23.1%, and 7.4%, respectively.The overall incidence ofneuropathy, nausea and vomiting, andrenal toxicity was similar in all threearms. Thus, triple combination chemotherapywas associated with an increasein overall toxicity and did nothave an efficacy advantage over twodrugcombinations. Furthermore, alternatingtwo-drug combinationsfailed to improve response or survivalcompared with a standard two-drugregimen.
The mitomycin, ifosfamide, andcisplatin (MIP) regimen, which iscommonly used in Europe, was notfound to be superior for survival inpatients with advanced non-small-celllung cancer compared with the doubletof gemcitabine/carboplatin.[22] Inthis study, 422 patients were randomizedto receive gemcitabine(1,200 mg/m2 on days 1 and 8) pluscarboplatin at AUC 5, or mitomycin(6 mg/m2 on day 1) plus ifosfamide(3 mg/m2 on day 1) andcisplatin (50 mg/m2 on day 1). Thegemcita-bine/carboplatin combinationwas better tolerated than the triplecombination but was associated withgreater grade 3/4 thrombocytopenia(8% vs 3%). While overall responserates were similar, median survival and1-year survival were improved withgemcitabine/carboplatin comparedwith the triple combination (10.0 vs6.5 months and 38% vs 40%, respectively;P = .0043). In summary, datado not suggest that three-drug combinationsor alternating two-drug combinationsare superior to two-drugcombinations in the treatment ofnon-mall-ell lung cancer.Chemotherapy in the ElderlyThe incidence of lung cancer ishighest in patients between the agesof 70 and 80. Due to a reluctance toadminister chemotherapy to elderlypatients because of lack of a perceivedbenefit and/or possible increased toxicity,presence of comorbidities,and/or poor performance status, elderlypatients have often not receivedchemotherapy. However, a retrospectiveanalysis of a randomized phase IIItrial of platinum-based chemotherapyregimens (ECOG 5592) was recentlyreported that compared outcomes ofpatients 70 years of age or older withthose of younger patients.[23] Elderlypatients who were physically fit wereable to tolerate platinum-based chemotherapyas well as younger patients.In the study, all patients were randomizedto one of a variety of platinumbasedtherapies and evaluated for responseand drug tolerance.Clinical response (partial or complete)to therapy was 21.5% inyounger patients and 23.3% in patients≥70 years of age. Median time to progressionwas 4.37 months in youngerpatients compared with 4.30 monthsin elderly patients, with 1-ear survivalof 38% and 29%, respectively, and2-ear survival of 14% and 12%, respectively.Both patient populationsshowed similar functional declines,with the elderly exhibiting a higherincidence of cardiovascular and respiratorycomorbidities and experiencingmore leukopenia and neuropsychiatrictoxicity. The authors concludedthat advanced age alone should notpreclude the use of chemotherapy forelderly non-mall-cell lung cancerpatients with good performance status.[23]It is unclear whether two-drug combinationsare preferred over singleagenttherapy in the elderly. Severalthird-generation chemotherapeuticagents appear to be effective in improvingsurvival of patients with advancednon-small-cell lung cancerover the age of 70. The trial by theElderly Lung Cancer VinorelbineStudy Group (ELVIS) demonstratedthat single-agent vinorelbine was superiorto best supportive care with significantlysuperior survival and qualityof life for elderly patients.[24] In aphase III trial conducted by the SouthernItaly Cooperative Oncology Group(SICOG), the combination ofvinorelbine and gemcitabine was superiorto vinorelbine alone in elderlypatients older than 70. Median survivalwith the combination was 21 weeks,compared with 18 weeks for singleagentvinorelbine.[25] The 1-year survivalrate was also superior in the two drugarm.These results conflicted with thosefrom the Multicenter Italian LungCancer in the Elderly Study (MILES),in which patients with advanced non-small-cell lung cancer were randomizedto receive one of three treatments:single-agent gemcitabine, single-agentvinorelbine, or the combination.[26]Early results indicated that bothgemcitabine and gemcitabine plusvinorelbine produced the best responserates (18.4%), and the trial wascontinued to a final enrollment of 700patients. However, further evaluationof all patients has shown that the combinationregimen provided no statisticallysignificant benefit with respectto median survival or 1-year survivalcompared with single-agent vinorelbineand was associated with moretoxicity. Hence, this study demonstratedthat the vinorelbine/gemcitabinecombination does not offer a significantadvantage over single-agentvinorelbine or gemcitabine.These and other similar results haveled investigators to believe that chemotherapyshould be considered forelderly patients with good performancestatus (0 or 1). Platinum-basedcombinations should be considered forthose elderly patients with a good performancestatus, but single agents maybe preferable for those patients withcomorbid diseases or who have performancestatus of 2.Novel Therapies for Advanced-Stage Non-Small-Cell LungCancerWith the increased use of platinumaswell as non-platinum-based firstlineregimens, the number of patientswho still have a good performance statusafter first-line therapy seeking additionaltherapy after relapse has increased,[27] while the number of second-line therapies is limited. Thus,identifying novel therapies for thetreatment of non-small-cell lung canceris critical to improve outcomes forthese patients. Given that some investigatorsbelieve a plateau has beenreached with cytotoxic chemotherapyin the treatment of advanced non-small-cell lung cancer, novel biologicbasedtherapies may provide furtheradvances by virtue of their uniquemechanisms of action (Table 3).While some of the molecularmechanisms underlying the pathologicprocesses of lung cancer remain unknown,several cellular and biologicadvances have identified a number ofmolecules and cellular pathways involved in the regulation of tumorgrowth and proliferation. In non-small-cell lung cancer, novel therapiesdirected against certain molecular targetsmay improve survival and qualityof life beyond that achieved withchemotherapeutic agents. Selectedbiological agents under investigationfor treatment of non-small-cell lungcancer are presented in Table 3. Someof the more actively researched molecular-based inhibitors include anti-epidermal growth factor receptor (anti-EGFR) inhibitors, antiangiogenesisagents, and antisense therapies, whichare discussed below.Epidermal Growth FactorReceptor Inhibitors
The epidermal growth factor receptor(EGFR, also known as ErbB1 orHER1) appears to play a critical rolein tumorigenesis because of its abundantexpression on cancer cells and itsability to stimulate cellular pathwaysthat increase cell proliferation, decreasecell death (apoptosis), and increaseangiogenesis.[28] Treatmentwith EGFR inhibitors is associatedwith a decrease in tumor cell proliferationand/or viability in vitro andin vivo.
- Gefitinib-Gefitinib (Iressa), oneof the first members in this new classof selective EGFR tyrosine kinase inhibitors,blocks EGFR activity(autophosphorylation) and subsequentsignal transduction mechanisms thatregulate proliferation, metastasis, andangiogenesis.[29] Gefitinib is orallyactive, and has been associated with alow side effect profile-primarily acneiformrash and diarrhea. Twomulticenter, randomized, dosecomparativephase II trials of gefitinibas monotherapy in patients withlocally advanced or metastatic non-small-cell lung cancer have been conducted.The Iressa Dose Evaluationin Advanced Lung Cancer Trial-1(IDEAL-1) was conducted in Japan,Europe, South Africa, and Australia.Eligible patients had received one ortwo previous chemotherapy regimens,one of which was to have been platinum-based.[30] The IDEAL-2 trial,conducted in the United States, requiredpatients to have received atleast two prior chemotherapy regimenscontaining a platinum agent anddocetaxel given separately or concurrently.[31]In the IDEAL-1 trial, 210 patientswere randomized to receive gefitinibat a daily oral dose of 250 mg(n = 103) or 500 mg (n = 106) (onepatient did not receive treatment at the250-mg dose).[30] Overall responserates of 18.4% and 19.0% were observedfor patients receiving the 250-and 500-mg doses, respectively. Forpatients who responded, median durationof response was more than 3months, and median survival timeswere 7.6 and 8.0 months for the 250-and 500-mg dose groups, respectively.In IDEAL-2, of the 216 patientsrandomized, 102 patients were treatedwith a daily oral dose of 250 mg ofgefitinib and 114 patients received adose of 500 mg of gefitinib.[31] Responseswere observed in 11.8% ofpatients treated with 250 mg ofgefitinib and 8.8% of patients treatedwith 500 mg. Median survival was 6.1and 6.0 months for patients in the 250-and 500-mg groups, respectively.Symptom improvement on theIDEAL trials occurred rapidly; themedian time to improvement for bothtreatment groups was 8 days inIDEAL-1, and 10 days and 9 days inthe 250- and 500-mg groups, respectively,in IDEAL-2. Symptom improvementoccurred in 40.3% and43.1% of patients who received 250-mg gefitinib in the IDEAL-1 andIDEAL-2 studies, respectively.[32]For the patients receiving 500 mg/d,37% of the IDEAL-1 participants and35.1% of the IDEAL-2 participantshad symptom improvement.[32] Inboth studies a substantially greaterproportion of patients with an objectivetumor response or stable diseaseshowed improvements in symptomscompared with patients who had progressivedisease. There was also apositive association between symptomresponse and survival; patients withimprovements in symptoms hadgreater survival. Based on the resultsof these clinical trials, particularlyIDEAL-2, gefitinib was approved foruse in the United States in the treatmentof patients with non-small-celllung cancer in the third-line setting.

- Improvements in quality of lifewere also seen in these trials. Overall,the quality-of-life improvement ratewas 23.9% and 21.9% for the 250- and500-mg/d dose groups, respectively,on IDEAL-1, and 34% and 23% forthe 250- and 500-mg/d dose groups,respectively, on IDEAL-2.[32] As withsymptom improvement, quality-of-lifeimprovement was recorded in a greaterpercentage of patients who achievedan objective response followed bythose with stable disease. These resultsindicate that treatment with singleagentgefitinib had a positive impacton symptoms and quality of life inpatients with advanced non-small-celllung cancer.Combination therapy with gefitinibhas also been evaluated in the IressaNSCLC Trials Assessing CombinationTreatment (INTACT-1 and INTACT-2). These two randomized, multicenterphase III trials were conducted in chemotherapy-naive patients to determineif the combination of gefitinib andchemotherapy would show additive orsynergistic antitumor effects. In INTACT-1, patients with stage III/IVnon-small-cell lung cancer were randomizedto treatment withgemcitabine at 1,250 mg/m2 administeredon days 1 and 8 and cisplatin at80 mg/m2 on day 1, or to the samechemotherapy regimen combined with250 or 500 mg/d of gefitinib. Patientsin INTACT-2 were randomized to receivecarboplatin at AUC 6 pluspaclitaxel at 225 mg/m2 administeredevery 3 weeks with or without the samedoses of gefitinib. Although the combinationof gefitinib and platinum chemotherapydoublets was well tolerated inboth studies, no statistically significantincrease in response rate, survival, ortime to worsening of symptoms wasdemonstrated with the addition of thisEGFR inhibitor.[33,34]
- Erlotinib-Erlotinib-Erlotinib (Tarceva) isa quinazoline small-molecule tyrosinekinase inhibitor that exhibits its effectat nanomolar concentrations. In aphase I study, patients with advancedsolid malignancies refractory to conventionalchemotherapy were treatedwith erlotinib.[35] Forty patients wereenrolled, four of whom had non-small-ell lung cancer. Diarrhea andskin rash were the primary toxicitiesthat precluded treatment with dosesgreater than 150 mg/d. Among patientswith advanced, platinum-refractorynon-small-cell lung cancer,14.3% had an objective response and28.6% had stable disease.[35]A phase II trial evaluating erlotinibat 150 mg/d enrolled 56 patients withprogressive, recurrent non-small-celllung cancer previously treated with aplatinum-based chemotherapy regimen.[36] Eight patients (14.3%)achieved response (one complete, sevenpartial; six confirmed at week 12 andbeyond), 16 patients (28.6%) had stabledisease lasting ≥ 12 weeks, and 28 patients(57.1%) had documented progressionof their underlying malignancy.Toxicities included rash and diarrhea,and were generally mild. Only a singlepatient required transient dose reductionsdue to skin toxicity.
- Cetuximab-Cetuximab (Erbitux)is a mouse-human chimerized monoclonalantibody that specifically bindsto the EGFR, thereby inhibiting downstreamsignal transduction pathways.[37] In preclinical in vivo andin vitro studies, cetuximab has beenshown to enhance radiosensitivity,promote radiation-induced apoptosis,decrease cell proliferation, inhibit radiation-induced damage repair, andinhibit tumor angiogenesis. Furthermore,cetuximab has been shown toenhance cytotoxicity when combinedwith various chemotherapeuticagents.[37]In the clinical setting, phase I studiesof cetuximab alone and in combinationwith cisplatin have been reported.[38] Baselga et al presented asummary of three initial studies, whichincluded a single-dose trial ofcetuximab (CP02-9401), a weeklymultiple-dose trial (once weekly for 4weeks) (CP02-9502), or weekly incombination with cisplatin (CP02-9503). In CP02-9503, patients wererestricted to those who had head andneck cancers (n = 16) and non-smallcelllung cancer (n = 6). A total of 52patients were treated in these threestudies. In the CP02-9401 and CP02-9502 studies, cetuximab was administeredintravenously at doses rangingfrom 5 to 100 mg/m2. In CP02-9503,cetuximab doses ranged from 5 to400 mg/m2 given weekly with additionof cisplatin, every 4 weeks, at 60mg/m2 1 hour following cetuximab administration.Initially, three patientswere treated with 100 mg/m2 ofcisplatin, but two experienced grade 3or higher toxicities; the dose was thereforereduced to 60 mg/m2.Overall, cetuximab was well tolerated,with five episodes of grade 3 orhigher toxicity occurring with 317doses of cetuximab. The most frequentadverse reactions included fever andchills, asthenia, transaminase elevations,nausea, and skin toxicities(flushing [4 cases], seborrheic dermatitis[1 case], and acneiform rash [6cases]).A phase II study of cetuximab incombination with docetaxel in chemotherapy-refractory patients with advancednon-small-cell lung cancerwas reported.[39] Cetuximab was administeredat 400 mg/m2 IV during thefirst week followed by 250 mg/m2IV weekly. Docetaxel was administered at 75 mg/m2 IV every 3 weeks.Twenty-five patients had been enrolledat the time of the report, and 20 patientswere evaluable for response. Preliminaryresults showed that after two cyclesof therapy, four patients had a partialresponse and six had stable disease.Toxicities included acneiform rash(grade 2/3) in five patients and febrileneutropenia (grade 2/3) in two patients.A phase II open-label, multicenter,nonrandomized study has been designedto evaluate the safety and toxicityof chemotherapy with cetuximabin patients with stage IIIA/B non-small-cell lung cancer. Other objectivesare to determine the survival ratewith this regimen in comparison to establishedregimens, and to determinethe potential correlation of EGFR expressionwith survival rate. In thisstudy, patients will initially receivecetuximab at 400 mg/m2 IV on day 1.Beginning on day 8, cetuximab at250 mg/m2 plus paclitaxel at 45 mg/m2 and carboplatin at AUC 2 will bedelivered weekly for 7 weeks duringconcurrent radiation therapy (63.0 Gyin 34 daily fractions over 7 weeks).Concurrent cetuximab plus chemotherapy/radiation therapy will be followedby 3 weeks of single-agentcetuximab at 250 mg/m2/wk. Patientsthen receive two cycles of paclitaxel at200 mg/m2 and carboplatin at AUC 6every 3 weeks, plus cetuximab at250 mg/m2/wk for 6 weeks. This studyplans to accrue a minimum of 70 patients.Angiogenesis Inhibitors
In addition to EGFR inhibitors,angiogenesis inhibitors are being studiedto evaluate their potential as antineoplasticagents. Because tumors requirenew blood vessels to grow andmetastasize, it is possible to target specificfactors associated with new bloodvessel growth and disease progressionand thus inhibit solid tumor development.A principle target is vascularendothelial growth factor (VEGF),thought to be critical in angiogenesis.In a multicenter, randomized, phase IItrial, the combination of humanizedmurine antibody to VEGF (bevacizumab[Avastin]) with paclitaxelplus carboplatin for patients with advancednon-small-cell lung cancer re-sulted in life-threatening hemoptysisin 6 of 67 patients, with 4 fatalities.[40]Four of these six patients had squamouscell histology. A subset analysis of 53patients with nonsquamous lung cancerwas later performed and comparedwith patients who received chemotherapyalone.[41]Data from the small subset showedimproved overall response rate (32%vs 12%) for patients receivingbevacizumab compared with those receivingchemotherapy alone at boththe 7.5- and 15-mg/kg doses. Mediantime to survival was also improved-61 and 77 weeks at the 7.5- and 15-mg/kg doses, respectively, vs 53 weeksfor controls-and toxicity appeared tobe reduced in patients treated withanti-VEGF antibody. These findingssuggest that bevacizumab can besafely combined with chemotherapyin nonsquamous non-small-cell lungcancer; however, confirmatory studiesare needed.The Eastern Cooperative OncologyGroup trial E4599 is conducting anongoing study comparing paclitaxelplus carboplatin alone vs the samechemotherapy combination withbevacizumab. Additional antiangiogenicagents in phase I trials includeendostatin (a 20-kD protein fragmentof collagen type XVIII) and humanrecombinant angiostatin (internal fragmentof plasminogen) as well as thematrix metalloproteinase inhibitorsmarimastat and metastat.Antisense Therapies
The creation of oligonucleotidesthat specifically bind sequences ofRNA has become a novel targetingmechanism for many tumor types, includingnon-small-cell lung cancer.ISIS 3521 (LY900003) is a 20-basepairDNA oligonucleotide that bindsto the mRNA of protein kinase C alpha(an enzyme involved in the growthand differentiation of cancer cells),inhibiting its transcription.[42] ISIS3521 was evaluated in a phase I/II trialin which it was administered in combinationwith paclitaxel andcarboplatin to 53 patients with advanced,untreated non-small-cell lungcancer. Toxicity consisted of neutropenia(16 patients grade 3; 14 grade4) and thrombocytopenia (9 patientsgrade 3; 4 grade 4). Among 48 patientseligible for efficacy, 42% had a partialor complete response while only17% had disease progression duringtreatment. At the time of the report,median survival was 19 months and1-year actuarial survival was 75%,suggesting that this antisense oligonucleotideimproves survival comparedwith chemotherapy alone.A phase III trial was recently reportedin which 616 patients with advancednon-small-cell lung cancerwere randomized to receive eitherpaclitaxel at 175 mg/m2 pluscarboplatin at AUC 6, both given onday 3, or the same regimen in combinationwith ISIS 3521 (125, 175, or225 mg/d IV on days 0-14).[43] However,the addition of the antisense compoundfailed to improve overall responserate, survival, or time to diseaseprogression, suggesting a lack ofefficacy of this agent in the advanceddiseasesetting. Studies evaluatingother antisense compounds for treatmentof non-small-cell lung cancer areongoing.ConclusionsChemotherapy will certainly continueto play a central role in the managementof patients with stage III/IVnon-small-cell lung cancer. Resultsfrom several randomized clinical trialshave shown a survival advantagefor the third-generation regimens butno consistently significant differencesin survival when these platinum-baseddoublets were compared. Elderly patientswith good performance statusshould receive platinum-based combinationchemotherapy, as would theiryounger counterparts. Elderly, less fitpatients may be considered for singleagentchemotherapy having a moretolerable side effect profile.Newer targeted therapies have beenexplored in the treatment of non-small-cell lung cancer and hold promiseto further improve survival andquality of life. In the IDEAL trials,treatment with gefitinib as monotherapyin heavily pretreated populationsof patients led to both objectiveresponses and symptom improvement.Although the INTACT studies failedto show a benefit of gefitinib in com-bination with platinum-based chemotherapydoublets in the first-line setting,further applications such as theuse of gefitinib in a maintenance approachfollowing primary treatmentfor locally advanced disease are beingexplored. Other agents, such aserlotinib, cetuximab, and bevacizumab,have also shown promise intreatment of this disease. These neweragents appear to potentiate the responseto cytotoxic therapy and mayprovide important therapeutic optionsfor patients with advanced non-smallcelllung cancer.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Cancer Facts and Figures. Atlanta, GA,American Cancer Society, 2003.
2.
American Thoracic Society/EuropeanRespiratory Society: Pretreatment evaluationof non-small-cell lung cancer. Am J Respir CritCare Med 156:320-332, 1997
3.
Humphrey EW, Smart CR, Winchester DP,et al: National survey of pattern of care of carcinomaof the lung. J Thorac Cardiovasc Surg100:837-843, 1990.
4.
Pignon JP, Stewart LA, Souhami RL, etal: A meta-analysis using individual patientdata from randomized clinical trials (RCTS) ofchemotherapy (CT) in non-small cell lung cancer(NSCLC): (2) survival in the locally advanced(LA) setting (abstract 1109). Proc AmSoc Clin Oncol 13:334, 1994.
5.
Clinical practice guidelines for the treatmentof unresectable non-small-cell lung cancer.Adopted on May 16, 1997, by the AmericanSociety of Clinical Oncology. J Clin Oncol15:1996-3018, 1997.
6.
Schaake-Koning C, van den Bogaert W,Dalesio O, et al: Effects of concomittantcisplatin and radiotherapy on inoperable nonsmall-cell lung cancer. N Engl J Med 326:524-530, 1992.
7.
Morton RF, Jett JR, McGinnis WL, et al:Thoracic radiation alone compared with combinedchemoradiotherapy for locallyunresectable non-small cell lung cancer. A randomized,phase III trial. Ann Intern Med115:681-686, 1991.
8.
Marino P, Pampallona S, Preatoni A, etal: Chemotherapy vs. supportive care in advancednon-small-cell lung cancer: Results ofa meta-analysis of the literature. Chest 106:861-865, 1994.
9.
Go RS, Adjei AA: Review of comparativepharmacology and clinical activity ofcisplatin and carboplatin. J Clin Oncol 17:409-422, 1999.
10.
Belani CP, TAX 326 Study Group:Docetaxel in combination with platinums(cisplatin or carboplatin) in advanced metastaticnon-small cell lung cancer. Semin Oncol29(suppl 12):4-9, 2002.
11.
Non-small Cell Lung Cancer CollaborativeGroup: Chemotherapy in non-small cell lungcancer: A meta-analysis using updated data onindividual patients from 52 randomized clinicaltrials. Br Med J 311:899-909, 1995.
12.
Perng RP, Chen YM, Ming-Liu J, et al:Gemcitabine versus the combination of cisplatinand etoposide in patients with inoperable nonsmall-cell lung cancer in a phase II randomizedstudy. J Clin Oncol 15:2097-2102, 1997.
13.
Manegold C, Bergman B, ChemaissaniA, et al: Single-agent gemcitabine versuscisplatin-etoposide: Early results of arandomised phase II study in locally advancedor metastatic non-small-cell lung cancer. AnnOncol 8:525-529, 1997.
14.
Vansteenkiste JF, Vandebroek JE,Nackaerts KL, et al: Clinical-benefit responsein advanced non-small cell lung cancer: Amulticentre prospective randomized phase IIIstudy of single agent gemcitabine versuscisplatin-vindesine. Ann Oncol 12:1221-1230,2001.
15.
Le Chevalier T, Brisgand D, DouillardJY, et al: Randomized study of vinorelbine andcisplatin versus vindesine and cisplatin versusvinorelbine alone in advanced non-small-celllung cancer: Results of a European multicentertrial including 612 patients. J Clin Oncol12:360-367, 1994.
16.
Masude N, Fukuoka M, Negoro S, et al:Randomized trial comparing cisplatin (CDDP)and irinotecan (CPT-11) versus CDDP andvindesine (VDS) versus CPT-11 alone in advancednon-small cell lung cancer (NSCLC)(abstract 1774). Proc Am Soc Clin Oncol18:459a, 1999.
17.
Schiller JH, Harrington D, Belani CP, etal: Comparison of four chemotherapy regimensfor advanced non-small-cell lung cancer.N Engl J Med 346:92-98, 2002.
18.
Belani CP, Langer C: First-line chemotherapyfor NSCLC: An overview of relevanttrials. Lung Cancer 38:S13-S19, 2002.
19.
Sederholm C: Gemcitabine (G) comparedwith gemcitabine plus carboplatin (GC)in advanced non-small cell lung cancer(NSCLC): A phase III study by the SwedishLung Cancer Study Group (SLUSG) (abstract1162). Proc Am Soc Clin Oncol 21:291a, 2002.
20.
Georgoulias V, Ardavanis A, AgelidouM, et al: Preliminary analysis of a multicenterphase III trial comparing docetaxel (D) versusdocetaxel/cisplatin (DC) in patients with inoperableadvanced and metastatic non-small celllung cancer (NSCLC) (abstract 1163). Proc AmSoc Clin Oncol 21:291a, 2002.
21.
Alberola V, Camps C, Provencia M, etal: Cisplatin/gemcitabine (CG) vs cisplatin/gemcitabine/vinorelbine (CGV) vs sequentialdoublets of gemcitabine/vinorelbine followedby ifosfamide/vinorelbine (GV/IV) in advancednon-small cell lung cancer (NSCLC): Resultsof a Spanish Lung Cancer Group Phase III Trial(GEPC/98-02) (abstract 1229). Proc Am SocClin Oncol 20:308a, 2001.
22.
Rudd RM, Gower NH, James LE, et al:Phase III randomised comparison ofmitomycin,ifosfamide and cisplatin (MIP) in advancednon-small cell lung cancer (NSCLC)(abstract 1164). Proc Am Soc Clin Oncol21:292a, 2002.
23.
Langer CJ, Manola J, Bernardo P, et al:Cisplatin-based therapy for elderly patientswith advanced non-small cell lung cancer: Implicationsof Eastern Cooperative OncologyGroup 5592, a randomized trial. J Natl CancerInst 94:173-181, 2002.
24.
Gridelli C: The ELVIS trial: A phase IIIstudy of single-agent vinorelbine as first-linetreatment in elderly patients with advancednon-small cell lung cancer. Elderly Lung CancerVinorelbine Italian Study. Oncologist6(suppl 1):4-7, 2001.
25.
Frasci G, Lorusso V, Panza N, et al:Gemcitabine plus vinorelbine yields better survivaloutcome than vinorelbine alone inelederly patients with advanced non-small celllung cancer. A Southern Italy Cooperative OncologyGroup (SICOG) phase III trial. LungCancer 34(suppl 4):S65-S69, 2001.
26.
Gridelli C, Cigolari S, Gallo C, et al:Activity and toxicity of gemcitabine andgemcitabine + vinorelbine in advanced nonsmall-cell lung cancer elderly patients: phaseII data from the Multicenter Italian Lung Cancerin the Elderly Study (MILES) randomizedtrial. Lung Cancer 31:277-284, 2001.
27.
Giaccone G: Clinical perspectives onplatinum resistance. Drugs 59(suppl 4):9-17,2000.
28.
Arteaga CL: Overview of epidermalgrowth factor receptor biology and its role as atherapeutic target in human neoplasia. SeminOncol 29(suppl 14):3-9, 2002.
29.
Raben D, Helfrich BA, Chan D, et al:ZD1839, a selective epidermal growth factorreceptor tyrosine kinase inhibitor, alone and incombination with radiation and chemotherapyas a new therapeutic strategy in non-small celllung cancer. Semin Oncol 29(suppl 4):37-46,2002.
30.
Fukuoka M, Yano S, Giaccone G, et al:Multi-institutional randomized phase II trial ofgefitinib for previously treated patients with advancednon-small-cell lung cancer. J ClinOncol 21:2237-2246, 2003.
31.
Kris MG, Natale RB, Herbst RS, et al: Aphase II trial of ZD1839 (SMQ-8216-SMQIressaSMQ-8217-SMQ) in advancednon-small cell lung cancer (NSCLC) patientswho had failed platinum- and docetaxel-basedregimens (IDEAL-2) (abstract 1166). Proc AmSoc Clin Oncol 21:292a, 2002.
32.
Cella D: Impact of ZD1839 on nonSMQ-8211-SMQsmallcell lung cancer-related symptoms as measuredby the Function Assessment of Cancer Therapy-Lung Scale. Semin Oncol 30:39-48, 2003.
33.
Giaccone G, Johnson DH, Manegold C,et al: A phase III clinical trial of ZD1839(SMQ-8216-SMQIressaSMQ-8217-SMQ) in combination with gemcitabine andcisplatin in chemotherapy-nave patients withadvanced non-small-cell lung cancer (INTACT1) (abstract 4O). Ann Oncol 13(suppl 5):2-3,2002.
34.
Johnson DH, Herbst R, Giaccone G, etal: ZD1839 (SMQ-8220-SMQIressaSMQ-8221-SMQ) in combination withpaclitaxel & carboplatin in chemotherapy-navepatients with advanced non-small-cell lungcancer (NSCLC): Results from a phase III clinicaltrial (INTACT 2) (abstract 468). Ann Oncol13(suppl 5):127-128, 2002.
35.
Hidalgo M, Siu LL, Neumanaitis J, etal: Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosinekinase inhibitor, in patients with advancedsolid malignancies. J Clin Oncol19:3267-3279, 2001.
36.
Perez-Soler R, Chachoua A, HubermanM, et al: A phase II trial of the epidermal growthfactor receptor (EGFR) tyrosine kinase inhibitorOSI-774, following platinum-based chemotherapyin patients (pts) with advanced, EGFRexpressing,non-small cell lung cancer(NSCLC) (abstract 1235). Proc Am Soc ClinOncol 20:310a, 2001.
37.
Kim ES, Khuri FR, Herbst RS: Epidermalgrowth factor receptor biology (IMCC225).Curr Opin Oncol 13:506-513, 2001.
38.
Baselga J, Pfister D, Cooper MR, et al:Phase I studies of anti-epidermal growth factorreceptor chimeric antibody C225 alone andin combination with cisplatin. J Clin Oncol18:904-914, 2000.
39.
Kim ES, Mauer AM, Fossella FV: Aphase II study of Erbitux (IMC-C225), an epidermalgrowth factor receptor (EGFR) blockingantibody, in combination with docetaxelin chemotherapy refractory/resistant patientswith advanced non-small cell lung cancer(NSCLC) (abstract 1168). Proc Am Soc ClinOncol 21:293a, 2002.
40.
DeVore R, Fehrenbacher L, Herbst R, etal: A randomized phase II trial comparingRhumab VEGF (recombinant humanizedmonoclonal antibody to vascular endothelialcell growth factor) plus carboplatin/paclitaxel(CP) to CP alone in patients with stage IIIB/IVNSCLC (abstract 1896). Proc Am Soc ClinOncol 19:485a, 2000.
41.
Johnson DH, DeVore R, Kabbinavar F, etal: Carboplatin (C) + paclitaxel (T) + RhuMab-VEGF (AVF) may prolong survival in advancednon-squamous lung cancer (abstract 1256). ProcAm Soc Clin Oncol 20:315a, 2001.
42.
Yuen A, Halsey J, Fisher G, et al: PhaseI/II trial of ISIS 3521, an antisense inhibitor ofPKC-alpha, with carboplatin and paclitaxel innon-small cell lung cancer (abstract 1234). ProcAm Soc Clin Oncol 20:309a, 2001.
43.
Lynch TJ, Raju R, Lind M, et al: Randomizedphase III trial of chemotherapy andantisense oligonucleotide LY900003 (ISIS3521) in patients with advanced NSCLC: Initialreport (abstract 2504). Proc Am Soc ClinOncol 22:623, 2003. Slide presentation availableat: http://www.asco.org/ac/1,1003,_12-002511-00_18-0023-00_19-003227-00_28-00RESULTPAGE,00.asp