Targeting the Insulin Growth Factor Pathway in Gastrointestinal Cancers
This review highlights the current status of the research in targeting the insulin growth factor pathway with a specific focus on gastrointestinal cancers.
Insulin-like growth factor 1 (IGF-1)–directed therapy is currently at a crossroads. After decades of research, several agents targeting the IGF pathway are now in clinical trials. One recent phase III trial of the IGF-1R inhibitor figitumumab in patients with non–small-cell lung cancer was discontinued after an interim analysis showed no survival improvement. Clinical trials for patients with sarcoma have demonstrated impressive anti-tumor activity in cases where the IGF-1 pathway is activated, such as in Ewing sarcoma; however, acquired resistance has been common. Recently, randomized phase II trials combining IGF-1R with epidermal grown factor receptor (EGFR) inhibition in colorectal cancer have been completed. Preclinical studies have indicated that several biomarkers may have potential predictive value. Studies of IGF-1R inhibitors in gastrointestinal cancers are currently ongoing in pancreatic, gastroesophageal, hepatocellular, and colorectal cancers. A critical analysis of prior work in this field and a rational strategy for maximizing success on the basis of biomarker use are necessary.
IGF-1R Targeting and Cancer
The insulin-like growth factor 1 (IGF-1) pathway is a key regulatory pathway that has been conserved in the evolutionary process and is responsible for cellular proliferation and survival in response to exogenous stimuli.[1] This role has particular relevance in cancer where cellular growth and survival despite apoptotic stimuli promote carcinogenesis and metastatic spread. Targeted inhibitors of the IGF-1 pathway, both monoclonal antibodies and small-molecule tyrosine kinase inhibitors, are currently under investigation in clinical trials for a diverse spectrum of cancers. This review highlights the current status of the research in this field with a specific focus on gastrointestinal cancers.
Physiology
The IGF-1 receptor (IGF-1R) is structurally similar to the insulin receptor (IR), with an 85% protein-sequence identity between the kinase domains of these receptors.[2,3] Both are transmembrane receptors with an extracellular, ligand-binding subunit and an intracellular α subunit, which has tyrosine kinase activity. In addition, the IGF-1 pathway consists of its ligands (IGF-1 and IGF-2), six binding proteins (IGFBP1-6) that limit the free or bioavailable ligand, its intracellular signaling proteins (insulin receptor substrates 1 and 2 (IRS-1 and IRS-2), and the downstream effector networks (the phosphatidylinositol 3-kinase [PI 3-kinase]/Akt/mTOR and the ras-raf-MAP kinase pathways).
IGF-1 is produced mainly in the liver and is controlled by human growth hormone (GH), which is secreted by the somatotrophic cells of the anterior pituitary.[4] The latter, in turn, are regulated by hypothalamic GH-releasing hormone and somatostatin. IGF-2 is also produced in the liver, and both these ligands activate IGF-1R. However, in the case of neoplastic transformation, cancer cells acquire autocrine or paracrine capacity for ligand production and may no longer be dependent on circulating ligand levels. Bergmann et al analyzed 12 pancreatic cancer specimens and noted a several-fold increase in IGF-1 mRNA transcripts in pancreatic cancer cells compared with normal pancreas or pancreatic cancer cell lines, suggesting that autocrine and paracrine IGF-1 production plays an important role in driving the IGF-1R.[5] Activation of IGF-1R results in the phosphorylation of IRS-1 and downstream effector proteins of the PI 3-kinase/Akt, mTOR, and S6 kinase pathways. IGF-2R, on the other hand, has no intracellular tyrosine kinase domain and therefore does not have a signaling role. Increased expression of IGF-2 in colon cancer (which results from the loss of imprinting [epigenetic silencing of one allele]) compared with the expression in normal colonic mucosa has underscored the role of IGF-2 in tumor progression.[6] The bioavailability of IGFs is limited by the six IGFBPs, of which IGFBP-3 has the greatest binding capacity. In the serum, the IGFs are bound to IGFBPs, which protect the ligands from proteolysis and thereby prolong their half-lives. It is believed that high levels of IGFBP-3 decrease the available IGF-1; however, the relationship is more complex and context-dependant. In certain cases, IGFBPs can actually increase IGF-1 signaling or can exert their effects in an IGF-1–independent fashion.
The role of the IGF-1 pathway in cancer development
Several mechanisms resulting from IGF-1R activation and signaling underlie oncogenesis and cancer progression. IGF-1R has several key features that suggest its role in regulating tumor growth; these include potent anti-apoptotic and mitogenic capacity, and a role in angiogenesis, invasion, and metastasis.[7] Support for a role for IGF-1R in oncogenic transformation is provided by the fact that IGF-1R–null fibroblasts do not undergo neoplastic transformation when exposed to cellular and viral oncogenes.[8] IGF-IR is not an oncogene; it is very rarely mutated in cancer. However, its expression is a requirement for neoplastic transformation by oncogenes such as K-ras.[9] IGF-1R–directed monoclonal antibodies and small molecules inhibit tumor growth and metastasis in xenograft models.[10,11] Recent preclinical studies have highlighted the role of the IGF-1 ligand in tumor invasiveness and metastases.[12] The levels of circulating IGF-1 in liver-specific IGF-1 gene-deleted (LID) mice are 75% lower than the levels in control mice; these lower levels result in smaller primary colon cancers and hepatic metastases.[13]
Human epidemiological studies
Several prospective studies have investigated the relationship between IGF-1 levels and the risk of developing cancer. A prospective nested case-control study within the Physicians’ Health Study reported a strong positive association between IGF-1 levels and prostate cancer risk. Men in the highest quartile of IGF-1 levels had a relative risk of 4.3 (95% confidence interval [CI], 1.8 to 10.6) compared with men in the lowest quartile.[14] Analysis of colon cancer risk in the Nurses’ and Physicians’ Health Studies indicated a high cancer risk in both women and men with the highest IGF-1 values. High levels of circulating IGF-1 and low levels of IGFBP-3 were independently associated with an elevated risk of colorectal cancer (CRC).[15,16] Several additional studies have been summarized in a large meta-analysis: in five studies in CRC, there was a positive association between elevated levels of circulating IGF-1 and CRC risk, with an odds ratio (OR) of 1.58 (95% CI, 1.11 to 2.27). On a multivariate meta-regression analysis, however, this association was of borderline statistical significance (P = .09).[17] In the European Prospective Investigation into Cancer and Nutrition (EPIC) study, however, serum concentrations of IGF-1 and IGFBP-3 showed no associations with CRC risk.[18] In another recently published meta-analysis, a modest positive association was reported between serum IGF-1 and CRC risk overall (relative risk = 1.07 for 1 standard deviation increase in IGF-I).[19] Genomic variations in the form of single nucleotide polymorphisms (SNPs) of the IGF pathway may also be associated with increased risk of gastrointestinal cancers. These are summarized in Table 1 and underscore the importance of this pathway in gastrointestinal malignancies.
Agents That Target IGF-1R
TABLE 1
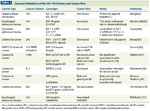
Genomic Variations of the IGF-1R Pathway and Cancer Risk
Monoclonal antibodies and small-molecule tyrosine kinase inhibitors that target IGF-1R are currently in phase II or III clinical trials for a variety of cancers. Other experimental, antagonistic options include antisense oligonucleotides, IGFBPs, and kinase-negative mutants. These agents are designed to block the expression and activation of IGF-1R and the ligand-receptor interactions. Over 30 agents have been developed for clinical investigation, and to our knowledge,12 are in clinical trials. The relevant trials in gastrointestinal cancers are shown in Table 2.
TABLE 2
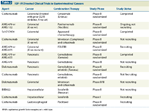
IGF-1R Directed Clinical Trials in Gastrointestinal Cancers
The antibodies block the ligand-receptor interaction, internalize the receptor, and cause the degradation of the receptor, while the tyrosine kinase inhibitors block activation of the receptor (tyrosine kinase activity) but not its expression. The present agents differ in their ability to target the insulin receptor (IR). Most tyrosine kinase inhibitors have a broader range and target IGF-1R, IR, and the hybrid receptor IGF-1R/IR. The antibodies do not generally bind to IR; however, figitumumab (CP-751,871) and Sch717454 may be able to block insulin signaling by binding to the hybrid receptor. Therefore, toxicity in the form of hyperglycemia is expected to differ between the antibodies and the tyrosine kinase inhibitors. Other secondary effects of IGF-1R inhibition include the elevation of IGF-1, growth hormone (GH), and insulin levels. This is particularly relevant in pancreatic and neuroendocrine cancers, in which glucose intolerance is common. In clinical trials, however, these effects have not led to serious toxicities, and the hyperglycemia can be easily managed with oral hypoglycemics like metformin or by insulin administration. Hyperinsulinemia resulting from IGF-1R inhibition raises theoretical concerns, since increased cancer risk and poor prognosis have been well documented in cancer patients with obesity and hyperinsulinemia.[1] However, to date there is no objective evidence of a deleterious effect in the cancers treated with IGF-1R inhibitors.
The monoclonal antibodies currently in clinical trials are either fully human or humanized, and hypersensitivity reactions are exceedingly rare. However, most antibodies are of the immunoglobulin G1 (IgG1) class, making them liable to induce antibody-dependant, cell-mediated cytotoxicity (ADCC) and complement fixation (the one exception is figitumumab, which is an IgG2 antibody). As a result, thrombocytopenia is more likely with the IgG1 antibodies. These toxicities have to be considered when planning combination therapies with cytotoxic agents.
Clinical Experience With IGF-1R Inhibition in Non-Gastrointestinal Cancers
Recent clinical experience with IGF-1R inhibition in the treatment of sarcomas and lung cancers provides valuable insights regarding the use of these agents for gastrointestinal cancers.
Sarcoma
Ewing sarcomas are characterized by a reciprocal (11;22)(q24;q12) translocation that generates an oncogenic fusion protein, EWS/FLI-1, which requires IGF-1R for transformation and which suppresses the expression of IGFBP-3. IGF-1R has been implicated in growth, metastasis, and angiogenesis in Ewing sarcoma and rhabdomyosarcoma, and IGF-1 activation is common in these malignancies.[20,21]
The largest study to be conducted in sarcoma patients was conducted by the Sarcoma Alliance for Research through Collaboration (SARC) global collaborative group. R1507, a human anti–IGF-1R IgG1 antibody, was given in a SARC phase II trial to 203 patients with recurrent or refractory sarcomas. Toxicities were few and included 15 severe adverse events in 9 patients. The most common toxicities were fatigue, thrombocytopenia, dehydration, and hyperglycemia. Clinically significant activity was observed in refractory Ewing sarcoma, rhabdomyosarcoma, and osteosarcoma. The response rate was 14%, and median overall survival was 17.6 months.[22,23] Clinical efficacy was also noted with other IGF-1R–targeted agents, including figitumumab, AMG 479, OSI-906 and BMS-754807. In Ewing sarcoma, impressive responses to IGF-1R inhibitors occurred in the refractory setting; however, these were infrequent (for instance, a 6% response rate with AMG 479), and acquired resistance was common with continued therapy.
Neuroendocrine tumors
IGF-1R expression is elevated in neuroendocrine tumors (NETs). The tyrosine kinase inhibitor NVP-AEW541 inhibited NET growth and led to apoptotic changes in the cancer cells.[24] Based on strong preclinical data, a phase II study of MK-0646 (dalotuzumab), an anti–IGF-1R antibody, was conducted; 25 patients with metastatic well-differentiated NETs (15 carcinoids, 10 islet cell carcinomas) were given MK-0646 as monotherapy. In this study, 5 patients achieved stable disease for 24 weeks or longer (range, 6.25 to 10.5 months). The most common serious adverse event possibly related to MK-0646 was hyperglycemia (7 patients, 25%).[25] The authors appropriately concluded that there was insufficient evidence of efficacy in their preliminary study to merit further investigation of IGF-1R inhibitors in NETs. However, this study did not include concurrent systemic chemotherapy, which can be effective in pancreatic islet cell carcinomas. Increasingly, it is being recognized that the IGF-1 pathway may not represent the sole mechanism for oncogenic transformation, given the absence of activating mutations; rather, the pathway may complement other mitogenic and anti-apoptosis/resistance mechanisms. Therefore, single-agent IGF-1R inhibitors may not be adequate therapy in most solid tumors.
Non–small-cell lung cancer (NSCLC)
In a phase Ib/II study of figitumumab in combination with standard doses of paclitaxel and carboplatin, two complete responses in patients with NSCLC were observed in the phase I portion of the study.[26] In the phase II portion of the study, 156 treatment-nave patients with stage IIIb/IV NSCLC were randomly assigned in a 2:1 ratio to receive either paclitaxel, carboplatin and figitumumab (PCI) or paclitaxel and carboplatin alone (PC); 54% of patients treated with PCI and 42% of patients treated with PC had objective responses. Sixteen of 23 patients assessable for efficacy in the nonrandomized single-arm extension cohort also responded to treatment. More responses occurred in patients with squamous cell histology (14 of 18 randomly assigned patients and 11 of 14 non-randomly assigned patients). Responses were also observed in two patients with squamous histology who received figitumumab on PC discontinuation. [27] Therefore a phase III trial was initiated with the same two arms in NSCLC. [28] In this trial, 88% had squamous cancer histology. Serious adverse events in the PCI arm included dehydration, hyperglycemia, and hemoptysis. Low pre-treatment body mass index and creatinine clearance were predictive of early death for patients receiving figitumumab. The trial was discontinued after 681 patients were recruited, as a result of the hazard ratio (HR) crossing the pre-specified futility boundary of 1.1 in favor of the PC arm. However, survival HR estimates favored PC in patients with low baseline levels of IGF-1 (minimum P = .006 for IGF-1 < 120 ng/mL; HR, 1.6) and favored PCI in those with high baseline levels of IGF-1 (minimum P = .13 for IGF-1 > 145 ng/mL; HR, 0.62). A phase III trial examining the effects of figitumumab in combination with erlotinib (Tarceva) as a second- or third-line treatment in patients with previously treated advanced NSCLC was also recently discontinued due to potential futility.
The above studies have provided some important lessons for the development of IGF-1R–targeted therapies in other cancer types, such as gastrointestinal cancers:
• Strong anti-tumor efficacy is seen in a limited population with IGF-1 pathway activation through mutations-eg, in Ewing sarcoma.
• Resistance or recurrence often follows response to IGF-1R–targeted agents.
• Experience in NSCLC suggests that multiple phenotypic and or molecular markers may be predictive of response (such as both squamous cell histology and high IGF-1 level).
• Single-agent IGF-1R inhibition may not be adequate, as noted in NET, and combination with chemotherapy may be needed.
• Combination strategies that target both EGFR and IGF-1R have not met with clinical success in NSCLC.
Gastrointestinal Cancers and IGF-1 Inhibition
Colorectal cancer
IGF-1R activation is crucial for the transforming activity of the epidermal growth factor receptor (EGFR). This interaction may be particularly important in CRC, in which EGFR inhibitors such as cetuximab (Erbitux) and panitumumab (Vectibix) are the standard of care.[29] In preclinical models of CRC, the combination of IGF-1R and EGFR inhibition resulted in enhanced growth arrest.[30] The results of a randomized phase II study in CRC evaluating IMC-A12 (cixitumumab) with or without cetuximab were recently published.[31] Patients with anti-EGFR antibody–refractory CRC (including mutant and wild-type [wt] K-ras tumors) were randomly assigned to treatment with either IMC-A12 as monotherapy or IMC-A12 in combination with cetuximab. The trial was further expanded, and an additional arm of patients with K-ras wt tumors was added after a patient with this genotype achieved a partial response to IMC-A12 plus cetuximab. Serious adverse events thought to be related to IMC-A12 included infusion-related reaction, thrombocytopenia, hyperglycemia, and pyrexia. Overall, 64 patients were enrolled in the three-arm study, and one patient demonstrated a partial response, with disease control lasting 6.5 months, but no additional antitumor activity was demonstrated. In 11 patients, correlative data were collected. Analysis of tumor samples by immunohistochemistry (IHC) revealed modest to strong IGF-1R staining in 3 of the 11 tumors. The one responding patient exhibited only low IGF-1R expression and no pAkt.[31] An additional large phase II/III study was performed in patients with irinotecan (Camptosar)-refractory CRC with K-ras wt who were treated with irinotecan and cetuximab ± MK-0646. The combination of cetuximab, irinotecan, and MK-0646 was well tolerated; efficacy data have not yet been published.[32]
IGF-1 expression was correlated with clinical outcomes in K-ras wt tumors treated with irinotecan and cetuximab. Progression-free survival in patients with IGF-1–negative tumors was superior to that in patients with IGF-1–positive tumors, suggesting that IGF-1 expression may be a prognostic factor for resistance in K-ras wt tumors treated with irinotecan and cetuximab.[33] IGF pathway polymorphisms were also associated with clinical outcomes in patients with K-ras wt tumors treated with cetuximab monotherapy.[34] These studies suggest that IGF-1 expression may identify a subgroup of patients with K-ras wt tumors who are likely to benefit from EGFR + IGF-1R–targeted treatments. However, no prospective study has yet assessed the effect of IGF-1R inhibition in CRC patients with high levels of IGF-1. Furthermore, most of the IGF-1R inhibitors currently in clinical trials are K-ras–independent; therefore, their activity in K-ras mutant CRC is also worthy of investigation.
Pancreatic cancer
Pancreatic adenocarcinoma has a dismal prognosis, with a 5-year survival rate of 5% for all stages combined. Gemcitabine (Gemzar) has been the mainstay of treatment for the last ten years despite limited efficacy. The EGFR inhibitor erlotinib (Tarceva) results in a survival benefit when added to gemcitabine. However, resistance is common through cross-talk between EGFR and the IGF-1R pathways.[35] Increased IGF-1 and IGF-1R expression occurs in a number of gastrointestinal cancers, including pancreatic cancer.[29] In vivo inhibition of IGF-1R expression decreased pancreatic cancer growth in an orthotopic model.[36] Our study of genetic variations in the IGF-1R pathway demonstrated that SNPs within this pathway correlate with worse overall survival in patients with pancreatic cancer.[37] The prognostic value of one SNP in IRS1 was validated in a retrospective cohort of 706 pancreatic cancer patients at our institution.
Three randomized phase II studies are currently investigating IGF-1R–directed monoclonal antibodies in combination with gemcitabine for patients with advanced pancreatic cancer. At the MD Anderson Cancer Center, the monoclonal antibody MK-0646 is being investigated in a three-arm randomized phase II study of gemcitabine + MK-0646, gemcitabine + erlotinib + MK-0646, and gemcitabine + erlotinib (control arm). Thus far, 55 patients have been enrolled (out of a planned 100) and 11 (20%) confirmed partial responses have occurred. Interestingly, these have been sustained partial responses (one as long as 62 weeks) and only one of these has been in the control arm. In a preliminary analysis, no significant survival improvement was noted with the addition of erlotinib. Kindler et al reported the results of a randomized phase II study of gemcitabine + AMG 479 (an IGF-1R–directed monoclonal antibody), gemcitabine + AMG 655 (antibody against death receptor 5 [DR5]), and gemcitabine + placebo.[38] In a final analysis of this phase II trial, the 12-month overall survival was 39%, 20%, and 23%, in the AMG 479, AMG 655, and control arms, respectively, with a HR of 0.65 in favor of the study arms. A phase III study of gemcitabine ± AMG 479 is currently underway. In both these studies, the toxicities of the study agent have been tolerable, with thrombocytopenia, hyperglycemia, and neutropenia being the principal toxicities (10% to 20% grade 3 or 4 events). The Southwest Oncology Group (SWOG) is investigating the monoclonal antibody IMC-A12 in a phase II randomized study of gemcitabine and erlotinib ± IMC-A12. Toxicity results of this study have been reported and appear to parallel the results of the above two studies. Further development of IGF-1R inhibitors in pancreatic cancer will depend on the results of the above studies.
Other clinical trials that are ongoing in gastrointestinal cancers include a trial of IMC-A12 in combination with sorafenib (Nexavar) for hepatocellular cancer (California Consortium) and a trial of paclitaxel ± IMC-A12 for metastatic esophageal cancer (Fox Chase Cancer Center). Efficacy data from these studies have not yet been reported.
While it is premature to draw conclusions from the above studies, it is evident that the combination of IGF-1R inhibitors with chemotherapy is tolerable and without serious toxicity. Hyperglycemia is not clinically significant even in diseases such as pancreatic cancer. In CRC, there does not appear to be a meaningful clinical synergism between the EGFR and the IGF-1R inhibitors.
IGF-1R Resistance
Based on the experience in soft tissue sarcomas as well as in pancreatic cancer, it is clear that acquired resistance to IGF-1R inhibitors is common and eventually leads to disease progression. There are several proposed mechanisms of resistance to the IGF-1R targeted agents, which can be summarized into two mechanistic groups.
The first group incorporates mechanisms that encourage the cells to adapt to alternative proliferative pathways by diminishing the activity of the IGF-1 pathway in cancer proliferation. Examples include the overexpression of IGFBPs, cross-talk between the IGF-1R and ErbB pathways, and HSP90.
• The bioavailability of circulating IGF-1 and IGF-2 is regulated by six IGFBPs as described earlier. Therefore, IGFBP overexpression has been implicated as a mechanism of resistance to the inhibition of IGF signaling. This has been demonstrated in vitro in sarcoma cell lines treated with BMS-536924 in which the overexpression of IGFBP-3 and IGFBP-6 correlated with resistance to IGF signaling inhibition.[39] Progressively increasing levels of IGFBPs have been noted in patients receiving IGF-1R inhibitors.[40] Whether this represents a resistance mechanism or simply a pharmacodynamic effect remains to be demonstrated.
• The IGF receptor family exhibits considerable crosstalk with the ErbB family of receptors (EGFR, HER2/neu, HER3, and HER4) and contributes to resistance to agents targeting these pathways. The co-inhibition of both pathways shows synergistic enhancement of apoptosis in several cancer cell lines. Data suggest that trastuzumab (Herceptin) resistance correlates with IGF-1R overexpression and thus that the co-inhibition of the IGF-1 family and the HER family may overcome this resistance.[41] The converse may also be true: in response to IGF-1R inhibition with MK-0646 and BMS-754807, HER2 expression was upregulated.[41,42] Similar data have been reported regarding HER3 activation after treatment with the IGF-1R–directed monoclonal antibody AVE1642. In this case, siRNA downregulation of HER3 resulted in increased sensitivity to AVE1642.[43]
• Transcription factors directly in control of IGF1R gene expression include tumor suppressor genes p53 and BRCA1. Wild type p53 suppressed IGF1R promoter activity in vitro by 90% while also suppressing endogenous IGF1R mRNA levels. Tumor-derived mutant forms of p53 enhanced IGF1R expression.[44] These preclinical experiments have demonstrated that lack of inhibition or even stimulation of the IGF1R promoter by mutant p53 may accelerate tumor growth. The BRCA1 gene product has also been shown to inhibit IGF1R gene expression.[45] Mutant BRCA1 may lead to dysregulated gene expression, with higher expression of IGF1R in patients with mutant BRCA1 breast cancer.[46]
• IGF-1R, Akt and Raf are dependant on the chaperone protein HSP90, which preserves their stability. HSP90 expression is increased in cancer cells, and its inhibition may be a strategy to block mitogenesis. In Ewing sarcoma cells, HSP90 expression is increased; its inhibition by siRNAs reduced cancer cell survival in IGF-1R–refractory cell lines.[47]
• Many tumors have chronically elevated activity of PI 3-kinase–dependent signaling pathways, caused largely by oncogenic mutation of PI 3-kinase itself or loss of the opposing tumor suppressor, PTEN. In cell lines (glioblastoma, breast, and prostate), PTEN loss caused desensitization of IGF/insulin signaling pathways, suggesting that the efficacy of inhibitors of IGF-1R could be limited in patients with PTEN loss or oncogenic mutation of PI 3-kinase.[48]
The second group of resistance mechanisms uses alternative IGF signaling by bypassing the IGF-1R pathway via the closely related IR or the hybrid IGF-1R/IR (HR).[49,50] Moreover, IR is expressed in many different tumor types, including colon.[51] If IGF-1R is blocked, IGF signaling can still occur through IRs or HRs.[52] For complete blockage of the IGF system, signaling through IGF-1R, IR, and HR must be inhibited. In animal models, dual IGF-1R and HR antibodies showed superior tumor inhibition compared to antibodies that target IGF-1R alone.[53]
Predictive Biomarkers
There is currently a critical need for the identification of biomarkers in clinical specimens that could potentially be used to stratify patients for IGF-1R–directed therapies. Fortunately, there exists a considerable body of preclinical data as well as clinical experience in other cancer types that will be invaluable for developing IGF-1R–targeted therapies in gastrointestinal cancers.
TABLE 3

Preclinical Studies of Potential Biomarkers of the IGF-1 Pathway
Zha et al investigated molecular predictors of the IGF-1R monoclonal antibody h10H5. They noted that in breast cancer and CRC, expression of IGF-1R is necessary for anti-tumor activity but by itself is not sufficient.[54] In their study, at least 1300 receptors per cell were needed for in vitro anti-tumor efficacy of h10H5. It is possible that oncogenic transformation requires expression of IGF-1R even at low levels, which may not be detectable by IHC. Therefore, IGF-1R expression by IHC may be a better negative predictor than positive predictor of response to anti–IGF-1R therapy, but this may not, by itself, have sufficient predictive value. Other preclinical studies (summarized in Table 3) suggest that activation of the IGF-1R pathway within cancer cells is required for anti-tumor efficacy of an agent targeting this pathway. For instance, IGF-1R needs IRS-1 in order to send mitogenic signals-and without IRS-1, IGF-1R sends a differentiation signal to the cells.[55] Thus, IRS-1 may be a requirement for IGF-1R activity and could potentially serve as a biomarker for sensitivity to agents that target IGF-1R. The Erk and Akt pathways are not activated by IGF-1R alone because of extensive cross-talk and may not accurately predict responsiveness to IGF-1R inhibition.
High IGF1R gene copy number was correlated with squamous cell histology and worse survival in lung cancer [56] Pitts et al, on the other hand, noted no IGF1R gene amplification in OSI-906–sensitive CRC cell lines.[57] But this OSI-906–sensitive line had an unbalanced gain of IGF1R based on ploidy. The cellular phenotype of IGF-1R–responsive cancer cells has not yet been defined. Squamous cell histology was considered as a possible phenotype that was responsive to IGF-1R inhibition. This was not proven to be the case in the figitumumab phase III study discussed above. Gualberto et al examined potential molecular and phenotypic determinants of figitumumab sensitivity in NSCLC. In their analysis, the mesenchymal phenotype was least likely to respond to IGF-1R inhibition.[58] However, this phenotype is also refractory to EGFR-targeted agents such as erlotinib,[59] and it remains to be determined whether mesenchymal differentiation is a prognostic marker rather than a predictive marker.
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
AMG 102
AMG 479
AMG 655
AVE1642
BIIB022
BMS-536924
BMS-754807
Carboplatin
Cetuximab (Erbitux)
Cixitumumab (IMC-A12)
Dalotuzumab (MK-0646)
Erlotinib (Tarceva)
Figitumumab
FOLFIRI (folinic acid, fluorouracil, and irinotecan)
Gemcitabine (Gemzar)
h10H5
Irinotecan (Camptosar)
NVP-AEW541
OSI-906
Paclitaxel
Panitumumab (Vectibix)
R1507
Sch717454
Sorafenib (Nexavar)
Trastuzumab (Herceptin
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Pharmacodynamic studies have investigated the modulation of the target pathways by IGF-1R inhibitors in clinical studies. Atzori et al investigated the modulation of pAkt, pMAPK, and pS6 kinase in paired biopsies of patients receiving the antibody MK-0646. They demonstrated significant inhibition of IGF-1R and IGF-1R signaling post-therapy in patients who received this therapy. Furthermore, elevation of IGF-1 levels were also noted secondary to IGF-1R inhibition.[60] However, paired tumor biopsies are not feasible in the vast majority of patients, and potential surrogate biomarkers that can be investigated in the blood may be invaluable in this setting. De Bono et al investigated the expression of IGF-1R on circulating tumor cells (CTC) in phase I trials of figitumumab as a single agent or with chemotherapy. Treatment with figitumumab alone decreased both total CTC count and IGF-1R expression. CTCs and IGF-1R–positive CTCs were most frequently detected in advanced prostate cancer patients. This non-invasive technique allows the evaluation of the therapeutic effect of targeted inhibitors on the IGF-1R–positive CTCs.[61] An obvious limitation is that CTCs are not easily detectable in all solid tumors, but this method may have implications in breast cancer and CRC.
Thus, the current data suggest that IGF-1R pathway activation within the tumor may predict responsiveness to specific inhibitors. In all probability, a reliable predictive panel for IGF-1R inhibitors will include multiple markers beyond just IGF-1R protein expression.
Summary
IGF-1R targeted therapy has been shown to be safe in many cancer types. Compelling preclinical data suggest that IGF-1 pathway inhibition may lead to clinical benefit in gastrointestinal cancers. Epidemiological studies have identified the IGF-1 pathway as an important risk factor for the development of several cancers. Experience with IGF-1R inhibitors in lung cancer and sarcoma has provided invaluable information that can be applied in the treatment of gastrointestinal malignancies. Preliminary data in CRC indicate a lack of clinically meaningful synergism between the IGF-1R and EGFR inhibitors in K-ras wt tumors. The activation of the IGF-1 pathway may also be predictive of response to the IGF-1R inhibitors. However, acquired resistance is common and not accounted for in the current clinical trials. The identification and validation of biomarkers of IGF-1 pathway activation in clinical samples must be emphasized so as to increase our chances of success with IGF-1R–targeted therapies in gastrointestinal cancers.
Financial Disclosure:Dr. Javle is a principal investigator on a clinical trial of MK-0646 (Merck).
References:
REFERENCES
1. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915-28.
2. Chitnis MM, Yuen JS, Protheroe AS, et al. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364-70.
3. Pollak M. Insulin-like growth factor physiology and neoplasia. Growth Horm IGF Res. 2000;10 Suppl
A:S6-7.
4. Zumkeller W. The role of the GH/IGF system in pituitary tumorigenesis. Horm Metab Res. 2003;35:664-6.
5. Bergmann U, Funatomi H, Yokoyama M, et al. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 1995;55:2007-11.
6. Cui H, Cruz-Correa M, Giardiello FM, et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753-5.
7. Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20-47.
8. Sell C, Rubini M, Rubin R, et al. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993;90: 11217-21.
9. Bruchim I, Attias Z, Werner H. Targeting the IGF1 axis in cancer proliferation. Expert Opin Ther Targets. 2009;13:1179-92.
10. Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339-53.
11. Sachdev D, Hartell JS, Lee AV, et al. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem. 2004;279:5017-24.
12. Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873-7.
13. Wu Y, Yakar S, Zhao L, et al. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030-5.
14. Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563-6.
15. Giovannucci E. Insulin-like growth factor-I and binding protein-3 and risk of cancer. Horm Res. 1999; 51 Suppl 3:34-41.
16. Giovannucci E, Pollak MN, Platz EA, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev. 2000;9:345-9.
17. Renehan AG ZM, Minder C, O'Dwyer ST, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346-53.
18. Rinaldi S, Cleveland R, Norat T, et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer.126:1702-15.
19. Rinaldi S CR, Norat T, Biessy C, et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer. 2010;126:1702-15.
20. Manara MC, Landuzzi L, Nanni P, et al. Preclinical in vivo study of new insulin-like growth factor-I receptor–specific inhibitor in Ewing's sarcoma. Clin Cancer Res. 2007;13:1322-30.
21. Scotlandi K, Avnet S, Benini S, et al. Expression of an IGF-I receptor dominant negative mutant induces apoptosis, inhibits tumorigenesis and enhances chemosensitivity in Ewing's sarcoma cells. Int J Cancer. 2002;101:11-6.
22. Pappo AS, Patel S, Crowley J, et al. Activity of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF1R), in patients (pts) with recurrent or refractory Ewing's sarcoma family of tumors (ESFT): Results of a phase II SARC study. ASCO Meeting Abstracts. 2010;28:10000-.
23. Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol.11:129-35.
24. Hopfner M, Baradari V, Huether A, et al. The insulin-like growth factor receptor 1 is a promising target for novel treatment approaches in neuroendocrine gastrointestinal tumours. Endocr Relat Cancer. 2006;13:135-49.
25. Reidy EH DL, Segal M, Saltz L. A phase II clinical trial of MK-0646, an insulin-like growth factor-1 receptor inhibitor (IGF-1R), in patients with metastatic well-differentiated neuroendocrine tumors (NETs). ASCO Meeting Abstracts May 20 2010: 4163. 2010.
26. Pollak M EP, Karp D, et al. Safety and tolerability of the anti-IGF-I receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in patients with advanced solid tumors. American Association for Cancer Research Annual Meeting, Los Angeles, CA, (abstr LB-343) of the study patients with advanced. 2007.
27. Karp DD, Paz-Ares LG, Novello S, et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27:2516-22.
28. Yee D. How to train your biomarker. Clin Cancer Res.16:3091-3.
29. Coppola D, Ferber A, Miura M, et al. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol. 1994;14: 4588-95.
30. Morgillo F, Kim WY, Kim ES, et al. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795-803.
31. Reidy DL, Vakiani E, Fakih MG, et al. Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 28:4240-6.
32. Watkins DJTJ, Schmoll HJ, Trarbach T, et al. A phase II study of the anti-IGFR antibody MK-0646 in combination with cetuximab and irinotecan in the treatment of chemorefractory metastatic colorectal cancer. J Clin Oncol. 2009;27:15s. (suppl; abstr 4127).
33. Scartozzi M, Mandolesi A, Giampieri R, et al. Insulin-like growth factor 1 expression correlates with clinical outcome in K-RAS wild type colorectal cancer patients treated with cetuximab and irinotecan. Int J Cancer. 2010;127:1941-7.
34. Winder T, Zhang W, Yang D, et al. Germline polymorphisms in genes involved in the IGF1 pathway predict efficacy of cetuximab in wild-type KRAS mCRC patients. Clin Cancer Res. 2010;16:5591-602.
35. Hakam A, Fang Q, Karl R, Coppola D. Coexpression of IGF-1R and c-Src proteins in human pancreatic ductal adenocarcinoma. Dig Dis Sci. 2003;48:1972-8.
36. Maloney EK, McLaughlin JL, Dagdigian NE, et al. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63:5073-83.
37. Dong X, Javle M, Hess KR, et al. Insulin-like growth factor axis gene polymorphisms and clinical outcomes in pancreatic cancer. Gastroenterology. 139:464-73, 73 e1-3.
38. Kindler HL, Richards DA, Stephenson J, et al. A placebo-controlled, randomized phase II study of conatumumab (C) or AMG 479 (A) or placebo (P) plus gemcitabine (G) in patients (pts) with metastatic pancreatic cancer (mPC). ASCO Meeting Abstracts.28: 4035.
39. Huang F, Greer A, Hurlburt W, et al. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69:161-70.
40. Lacy MQ, Alsina M, Fonseca R, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol. 2008;26:3196-203.
41. Haluska P, Carboni JM, TenEyck C, et al. HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol Cancer Ther. 2008;7:2589-98.
42. Hendrickson AW, Haluska P. Resistance pathways relevant to insulin-like growth factor-1 receptor-targeted therapy. Curr Opin Investig Drugs. 2009; 10:1032-40.
43. Desbois-Mouthon C, Baron A, Blivet-Van Eggelpoel MJ, et al. Insulin-like growth factor-1 receptor inhibition induces a resistance mechanism via the epidermal growth factor receptor/HER3/AKT signaling pathway: rational basis for cotargeting insulin-like growth factor-1 receptor and epidermal growth factor receptor in hepatocellular carcinoma. Clin Cancer Res. 2009;15:5445-56.
44. Werner H, Karnieli E, Rauscher FJ, LeRoith D. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc Natl Acad Sci U S A. 1996;93:8318-23.
45. Abramovitch S, Glaser T, Ouchi T, Werner H. BRCA1-Sp1 interactions in transcriptional regulation of the IGF-IR gene. FEBS Lett. 2003;541:149-54.
46. Maor S, Yosepovich A, Papa MZ, et al. Elevated insulin-like growth factor-I receptor (IGF-IR) levels in primary breast tumors associated with BRCA1 mutations. Cancer Lett. 2007;257:236-43.
47. Martins AS, Ordonez JL, Garcia-Sanchez A, et al. A pivotal role for heat shock protein 90 in Ewing sarcoma resistance to anti-insulin-like growth factor 1 receptor treatment: in vitro and in vivo study. Cancer Res. 2008;68:6260-70.
48. Lackey J BJ, Davidson L, Batty IH, Leslie NR, Downes CP. Loss of PTEN selectively desensitizes upstream IGF1 and insulin signaling. Oncogene. 2007;26:7132-42.
49. Frasca F, Pandini G, Sciacca L, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23-37.
50. Pandini G, Frasca F, Mineo R, et al. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002; 277:39684-95.
51. Denley A, Wallace JC, Cosgrove LJ, Forbes BE. The insulin receptor isoform exon 11- (IR-A) in cancer and other diseases: a review. Horm Metab Res. 2003;35: 778-85.
52. Doepfner KT, Spertini O, Arcaro A. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia. 2007;21:1921-30.
53. Pandini G, Wurch T, Akla B, et al. Functional responses and in vivo anti-tumour activity of h7C10: a humanised monoclonal antibody with neutralising activity against the insulin-like growth factor-1 (IGF-1) receptor and insulin/IGF-1 hybrid receptors. Eur J Cancer. 2007;43:1318-27.
54. Zha J, O'Brien C, Savage H, et al. Molecular predictors of response to a humanized anti-insulin-like growth factor-I receptor monoclonal antibody in breast and colorectal cancer. Mol Cancer Ther. 2009; 8:2110-21.
55. Baserga R. The insulin receptor substrate-1: a biomarker for cancer? Exp Cell Res. 2009;315:727-32.
56. Dziadziuszko R, Merrick DT, Witta SE, et al. Insulin-like growth factor receptor 1 (IGF1R) gene copy number is associated with survival in operable non-small-cell lung cancer: a comparison between IGF1R fluorescent in situ hybridization, protein expression, and mRNA expression. J Clin Oncol.28:2174-80.
57. Pitts TM, Tan AC, Kulikowski GN, et al. Development of an integrated genomic classifier for a novel agent in colorectal cancer: approach to individualized therapy in early development. Clin Cancer Res.16:3193-204.
58. Gualberto A, Dolled-Filhart M, Gustavson M, et al. Molecular analysis of non-small cell lung cancer (NSCLC) identifies subsets with different sensitivity to insulin like growth factor I receptor (IGF-IR) inhibition. Clin Cancer Res. 2010;16:4654-65.
59. Yauch RL, Januario T, Eberhard DA, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;11:8686-98.
60. Atzori F, Tabernero J, Cervantes A, et al. A phase I, pharmacokinetic (PK) and pharmacodynamic (PD) study of weekly (qW) MK-0646, an insulin-like growth factor-1 receptor (IGF1R) monoclonal antibody (MAb) in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts. 2008;26:3519.
61. de Bono JS, Attard G, Adjei A, et al. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin Cancer Res. 2007;13:3611-6.
62. Weng CJ HY, Tsai CM, Chu YH, et al. Relationship of insulin-like growth factors system gene polymorphisms with the susceptibility and pathological development of hepatocellular carcinoma. Ann Surg Oncol. 2010;17:1808-15.
63. MacDonald K PG, Guernsey DL, Zhao R, Casson AG. A polymorphic variant of the insulin-like growth factor type I receptor gene modifies risk of obesity for esophageal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2009;33:37-40.
64. Suzuki H LY, Dong X, Hassan MM, et al. Effect of insulin-like growth factor gene polymorphisms alone or in interaction with diabetes on the risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3467-73.
65. Wong HL DK, Probst-Hensch N, Koh WP, et al. A new single nucleotide polymorphism in the insulin-like growth factor I regulatory region associates with colorectal cancer risk in Singapore Chinese. Cancer Epidemiol Biomarkers Prev. 2005;14:144-51.
66. Zecevic M AC, Gu X, Campos IM, Jones JS, et al. IGF1 gene polymorphism and risk for hereditary nonpolyposis colorectal cancer. J Natl Cancer Inst. 2006;98:139-43.
67. Slattery ML SW, Curtin K, Ma KN, et al. Associations among IRS1, IRS2, IGF1, and IGFBP3 genetic polymorphisms and colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1206-14.
68. Le Marchand L KL, Henderson BE, Wilkens LR. Association of an exon 1 polymorphism in the IGFBP3 gene with circulating IGFBP-3 levels and colorectal cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2005 14:1319-21.
69. Khoury-Shakour S GS, Lejbkowicz F, Rennert HS, et al. Recreational physical activity modifies the association between a common GH1 polymorphism and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:3314-8.
70. Pechlivanis S WK, Chang-Claude J, Hoffmeister M, et al. Polymorphisms in the insulin like growth factor 1 and IGF binding protein 3 genes and risk of colorectal cancer. Cancer Detect Prev. 2007;31:408-16.
71. McElholm AR MA, Patterson CC, Johnston BT, et al; Finbar Group. A population-based study of IGF axis polymorphisms and the esophageal inflammation, metaplasia, adenocarcinoma sequence. Gastroenterology. 2010;139:204-12.
72. Zha J, Lackner MR. Targeting the insulin-like growth factor receptor-1R pathway for cancer therapy. Clin Cancer Res.16:2512-7.
73. Mukohara T, Shimada H, Ogasawara N, et al. Sensitivity of breast cancer cell lines to the novel insulin-like growth factor-1 receptor (IGF-1R) inhibitor NVP-AEW541 is dependent on the level of IRS-1 expression. Cancer Lett. 2009;282:14-24.
74. Cao L, Yu Y, Darko I, et al. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;68:8039-48.