Targeting Metastatic Colorectal Cancer in 2008: A Long Way From 5-FU
Colorectal cancer is one of the leading causes of cancer-related death worldwide, with almost 20% of all patients presenting with metastatic disease at the time of their diagnosis. The treatment regimens and options of metastatic colorectal cancer have significantly changed in the last 10 years, leading to an improvement of response rates to about 50%, progression-free survival of about 10 months, and overall survival reaching over 2 years.
ABSTRACT: ABSTRACT:Colorectal cancer is one of the leading causes of cancer-related death worldwide, with almost 20% of all patients presenting with metastatic disease at the time of their diagnosis. The treatment regimens and options of metastatic colorectal cancer have significantly changed in the last 10 years, leading to an improvement of response rates to about 50%, progression-free survival of about 10 months, and overall survival reaching over 2 years. Beside US Food and Drug Administration approval of the cytotoxic agents irinotecan (Camptosar), oxaliplatin (Eloxatin), and capecitabine (Xeloda), the increasing understanding of molecular pathways that comprise the cell cycle, apoptosis, angiogenesis, and invasion has provided novel targets in cancer therapy. The biologic agent bevacizumab (Avastin), an inhibitor against vascular endothelial growth factor, and cetuximab (Erbitux) and panitumumab (Vectibix), monoclonal antibodies to epidermal growth factor receptor, have demonstrated their efficacy in clinical trials. This article reviews the mechanisms of action and possible markers of resistance, and summarizes data on the clinical efficacy of targeting agents.
Colorectal cancer (CRC) is one of the leading causes of cancer-related death worldwide. With an estimated 153,760 newly diagnosed cases and 52,180 cancer-related deaths in the United States for 2007, it will remain the second leading cause of cancer-related death.[1] Almost 20% of these patients will present at the time of diagnosis with metastatic disease (mCRC).[2] For nearly 35 years, the standard chemotherapeutic regimen for treating metastatic colorectal carcinoma consisted only of fluorouracil (5-FU), with an overall response rate of 10% and a median survival of 10 months.[3,4] With the addition of leucovorin to 5-FU, the response rate improved to 23%.[5] During the past 10 years, with the addition of the cytotoxic agents irinotecan (Camptosar) and oxaliplatin (Eloxatin), a substantial change in response rate, progression-free survival, and overall survival occurred.
Furthermore, the increasing understanding of molecular pathways that characterize the cell cycle, apoptosis, angiogenesis, and invasion has provided novel targets in cancer therapy.[6] The biologic agents bevacizumab (Avastin) as an inhibitor against vascular endothelial growth factor (VEGF), and cetuximab (Erbitux), a monoclonal antibody to the epidermal growth factor receptor (EGFR), received US Food and Drug Administration (FDA) approval for the treatment of metastatic CRC.
This article reviews the mechanisms of action, promising new agents, and markers of resistance, and summarizes data on the clinical efficacy of targeting agents.
Targeting VEGF
The revelation that tumor growth is angiogenesis-dependent identified new targets for anticancer treatment.[7,8] The promotion of new blood vessel formation is a tightly regulated complex, working via secretion of proangiogenic factors by tumor cells and adjacent stromal cells that activate endothelial cells.[9] Among these, the most prominent proangiogenic factors are VEGF, platelet-derived growth factor (PDGF), and angiopoietin-1.[9]
VEGF is a heparin-binding glycoprotein family that includes six members, referred to as VEGF-A through VEGF-E and PDGF.[10] VEGF is the most critical regulator for the development of neoangiogenesis and functions as an endothelial cell mitogen. It increases microvascular permeability, induces endothelial cell migration and division, reprograms gene expression, promotes endothelial cell survival, prevents senescence, and induces angiogenesis and lymphangiosis.[11]
VEGF-A through -E and PDGF act through specific binding to three different cell membrane receptors (VEGFR-1, -2, and-3), which consist of an extracellular domain, a transmembrane domain, and an intracellular region containing a tyrosine kinase domain. Binding of a ligand to its receptor induces the activation of the tyrosine kinase domain, which initializes the activation of intracellular signaling transduction pathways that are involved in regulation of cellular proliferation and survival, such as the raf/MEK, ERK, AKT, mTOR, IGFIR, and PI3K pathways.[12]
High VEGF serum levels have been found to be associated with poor outcome in cancer patients.[13,14] Numerous angiogenesis inhibitors (eg, bevacizumab, sunitinib [Sutent], vatalanib) have been developed in the clinic as antiangiogenic approaches to therapy. Inhibition of VEGF-A by bevacizumab in patients with mCRC appears to be the most promising such strategy so far.
Bevacizumab
Bevacizumab was the first angiogenesis inhibitor to demonstrate prolonged survival in patients with metastatic colorectal cancer by inhibition of all active isoforms of VEGF-A. A randomized phase III trial (AVF2107) with 813 previously untreated metastatic colorectal cancer patients showed that the addition of bevacizumab to IFL (irinotecan, bolus 5-FU, leucovorin) increased response rates as well as progression-free and overall survival. The toxicity profile revealed an increase in (bevacizumab-dependent) hypertension, but this was manageable with standard oral antihypertensive agents.[15] Today, bolus administration of 5-FU is no longer considered standard treatment. Recently, bevacizumab has been combined with the infusional regimens FOLFOX (leucovorin, 5-FU, oxaliplatin) and FOLFIRI (leucovorin, 5-FU, irinotecan).[16]
A phase III trial in the first-line setting (NO16966) showed that the addition of bevacizumab to FOLFOX4 or XELOX (capecitabine [Xeloda], oxaliplatin) resulted in an improved progression-free survival of 9.4 months, compared to 8 months with FOLFOX4 or XELOX (P < .0023). However, the addition of bevacizumab to chemotherapy showed no increase in overall survival (21.3 vs 19.9 months) or response rate (47% vs 49%).[17]
Interestingly, both studies showed an increase in progression-free survival in the bevacizumab arms, but the improvement in survival in NO16966 was unexpectedly small. One explanation for this finding is that chemotherapy was completely stopped in patients with neurotoxicity and was not continued with 5-FU/leucovorin and bevacizumab, which may have had an impact on progression-free survival. The duration of treatment with bevacizumab was 2.8 months longer in AVF2107 compared to NO16966, since patients did not experience dose-limiting toxicities such as the neurotoxicities seen with IFL treatment.
Surprisingly, there was no difference in response rate in NO16966 when bevacizumab was added to treatment. It remains unknown whether the mechanisms of action of bevacizumab including normalization of blood flow and therefore increased drug exposure, are clinically significant for the improvement in response rate.
Bevacizumab was also tested in chemorefractory patients. The Eastern Cooperative Oncology Group (ECOG) E3200 trial compared overall survival, progression-free survival, response rate, and toxicity in mCRC patients previously treated with a fluoropyrimidine and irinotecan. Results demonstrated that the combination-therapy with FOLFOX4 and bevacizumab improved survival duration (12.9 months with FOLFOX4/bevacizumab vs 10.8 months with FOLFOX4 vs 10.2 months with bevacizumab alone, P = .0011).This was the first study to demonstrate that antiangiogenic therapy also has a role in second-line therapies.
These findings raise the question of whether we should continue using bevacizumab in second-line treatment when patients experience disease progression on bevacizumab in the first-line setting? In a randomized study of the BRiTE registry (Bevacizumab Regimens Investigation of Treatment Effects and Safety), data suggest that continuation of bevacizumab therapy is associated with a longer overall survival. However, these data are retrospective and need to be validated. The Intergroup study S0600 will assess these data in a large prospective, randomized phase III investigation.
Another important question is: Should we develop additional, more effective antiangiogenic approaches?
Sunitinib
Several other drugs inhibiting angiogenesis are being tested for a variety of cancers in clinical trials. Sunitinib, a tyrosine kinase inhibitor against VEGF-1, -2, -3, PDGF, and fms-like tyrosine kinase,[18] showed impressive results in patients with metastatic renal cell carcinoma, including improvement in response (up to 40%) and doubling of progression-free survival up to 11 months.[19] However, a phase II trial with sunitinib in patients with mCRC showed no meaningful objective response rate,raising questions about the role of VEGFR tyrosine kinase inhibitors in colon cancer.[20]
Clinical trials of sunitinib in combination with chemotherapy for patients with mCRC are ongoing. Why sunitinib is successful in metastatic renal cell carcinoma (mRCC) and not in mCRC remains unsolved. One possible explanation might be that VEGF is more critical for angiogenesis in mCRC compared to mRCC.
Vatalanib
Vatalanib (PTK/ZK222584) is another tyrosine kinase inhibitor of VEGFR-1, -2, and -3–mediated angiogenesis and thereby reduces tumor growth and metastasis.[18] CONFIRM-1, a randomized phase III trial, assessed chemonaive mCRC patients treated with either FOLFOX/vatalanib or FOLFOX alone; the combination therapy reduced tumor permeability and vascularity.[21] However, treatment with vatalanib failed to show significant improvement in progression-free survival in the FOLFOX/vatalanib group. Interestingly, improvement in progression-free survival was seen in patients with high levels of serum lactate dehydrogenase (LDH). CONFIRM-2 randomized patients to second-line therapy with the same agents and confirmed the primary findings of response in patients with high LDH, indicating LDH as a potential marker of response to vatalanib therapy.[22]
Targeting EGFR
EGFR belongs to the ErbB or HER family and comprises four different transmembrane receptor kinases-HER1 or EGFR, HER2/neu, HER3, and HER4. Each receptor has an extracellular domain, a transmembrane region, and an intracellular region consisting of a tyrosine kinase domain.[2] Endogenous ligands of these receptors include EGF, transforming growth factor (TGF)-alpha, amphiregulin, betacellulin, and epiregulin. Probably the most important ligands are EGF and TGF-alpha, but recent data on epiregulin and amphiregulin suggest that expression levels may predict outcome in patients treated with cetuximab.[23] Activation of EGFR through one of the ligands results in intracellular signal transduction via the Ras/raf/MEK/ERK1/2 or PI3K/PTEN/Akt pathways.[24] All members of the ErbB family have been found to be important mediators of normal epithelial cell growth, differentiation, and cell survival; findings of upregulation of these components in cancer patients are therefore not surprising.[2]
Overexpression or upregulation of EGFR has been found in various malignancies, including head and neck, non–small-cell lung, breast, prostate, gastric, pancreatic, and ovarian cancers,[25-27] as well as in 60% to 80% of colorectal cancers.[26,28,29] This overexpression has been thought to be responsible for cell proliferation, invasion, angiogenesis, and metastasis,[30] and is related to cancer progression and a poor outcome.[31]
Two classes of EGFR inhibitors are clinically available; they inhibit either the extracellular domain (cetuximab, panitumumab [Vectibix]) or the intracellular tyrosine kinase domain (gefitinib [Iressa], erlotinib [Tarceva]). Erlotinib received FDA approval for the treatment of locally advanced or metastatic non–small-cell lung cancer and, in combination therapy with gemcitabine (Gemzar), for the treatment of unresectable or metastatic pancreatic cancer.
Monoclonal Antibodies
Cetuximab is a chimeric IgG1 monoclonal antibody directed against the ligand binding site of EGFR. After two published registration trials,[32,33] this agent was approved by FDA in 2004 for the treatment of EGFR-expressing mCRC, alone or in combination with irinotecan in patients not responding to irinotecan- or oxaliplatin-based chemotherapy, or as a single agent in those intolerant to irinotecan.
Furthermore, efficacy of cetuximab in combination with FOLFIRI has been demonstrated in newly diagnosed patients with mCRC in the CRYSTAL trial. A total of 1,217 patients with EGFR-positive tumors were randomized to either FOLFIRI/cetuximab or FOLFIRI alone. Results demonstrated a significantly longer progression-free survival (8.9 vs 8 months, P = .036) and a higher response rate (46.9% vs 38.7%, P = .005) in patients who received FOLFIRI plus cetuximab.[34]
Cetuximab has also been tested in second-line therapies. The phase III Erbitux Plus Irinotecan in Colorectal Cancer (EPIC) trial randomized 1,298 EGFR-positive patients with disease progression on an oxaliplatin-based first-line regimen to irinotecan alone or with cetuximab.[35] A significant improvement in progression-free survival and response rate was found with the combination compared to irinotecan alone (3.98 vs 2.56 months, P < .0001, 16% vs 4%).[2] These data show that EGFR inhibition demonstrated a consistent increase in efficacy in both first- and second- line therapies.
The BOND-2 trial was the first to combine bevacizumab and cetuximab to evaluate the efficacy and toxicity profiles of concurrent administration of bevacizumab/cetuximab with or without irinotecan in 83 bevacizumab- and cetuximab-naive patients in salvage therapy. Treatment with cetuximab, bevacizumab, and irinotecan produced a prolonged time to progression (7.3 vs 4.9 months) and increased response rate (37% vs 20%) compared to cetuximab/bevacizumab alone.[36] This study was the first to show a significant improvement in response rate, time to progression, and overall survival through combination of targeted agents without overlapping toxicities.
After these promising results, the PACCE trial was designed to assess efficacy through combination of VEGF and EGFR inhibition in the first-line setting. This phase III trial evaluated the benefit of adding panitumumab to either an oxaliplatin/bevacizumab-based (n = 823) or irinotecan/bevacizumab-based (n = 230) chemotherapeutic regimen in patients with mCRC. Panitumumab is a fully humanized IgG2 monoclonal antibody against the EGFR, which received FDA approval in 2006 for second-line therapy in the treatment of mCRC.[2]
The results of the PACCE trial showed that treatment with oxaliplatin/bevacizumab plus panitumumab was associated with a decreased progression-free survival (9.5 vs 11 months) and overall survival (19.3 vs 20.6 months) and increased toxicity, indicating that combination-therapy with oxaliplatin, bevacizumab, and panitumumab is associated with an unfavorable risk-benefit profile.[37] Patients treated with irinotecan/bevacizumab with or without panitumumab showed a similar median progression-free survival (10.6 vs 10.7 months) and overall survival (20.7 vs 20.5 months).[38]
Interestingly, results from the BOND-2 study clearly demonstrate that even in third-line therapy, the addition of cetuximab to bevacizumab/irinotecan produces an advantage in progression-free and overall survival, whereas the addition of panitumumab to either an oxaliplatin- or irinotecan-based regimen produces an unfavorable effect.This raises the question of why the combination of targeting agents for VEGF and EGFR was successful in third-line therapy, but not in the first line.
Two possible explanations include: (1) Patients and tumors are different in the first- and third-line settings, as exposure to chemotherapy and targeted agents may change the genetic makeup of the tumor and, therefore, change chemosensitivity. (2) Differences between the structure and mechanisms of action between these antibodies come into play. In contrast to the IgG2 monoclonal antibody panitumumab, cetuximab may produce cytotoxicity through immunologic mechanisms specific to IgG1 subtypes[39]-the so-called antibody-dependent cell-mediated cytotoxicity (ADCC). Panitumumab has a significantly greater EGFR-binding affinity, more likely causing an increase in skin and gastrointestinal toxicity, which may limit its potential for combination with cytotoxic agents.
Tyrosine Kinase Inhibitors
Few results from clinical trials are available for treatment with tyrosine kinase inhibitors of EGFR in mCRC patients. Gefitinib, which received FDA approval for treatment in metastatic or locally advanced non–small-cell lung cancer, was given in combination with FOLFOX4 as first-line treatment in patients with mCRC.[40] With a progression-free survival of 7.8 months and an overall survival of 13.9 months gefitinib failed to show a survival benefit in comparison to other first-line regimens.[32,35]
Interim efficacy results from a randomized phase II study of capecitabine/erlotinib or either agent alone showed that the combination therapy in the first-line setting is as least as active as either monotherapy.[41] The DREAM-OPTIMOX3-study was designed to evaluate the efficacy of VEGF inhibition (with bevacizumab) with or without EGFR inhibition (with erlotinib) plus either FOLFOX7 or XELOX in chemonaive mCRC patients. Results of this feasability study showed grade 3/4 side effects in 70% of patients receiving erlotinib in addition to bevacizumab/XELOX (the most common toxicity was diarrhea). These data suggest that EGFR tyrosine kinase inhibitors lack efficacy in mCRC and increase toxicity when combined with bevacizumab.[42]
Molecular Markers for Response Prediction
Although overall survival in mCRC patients has nearly doubled during the past 10 years, the question of why some patients respond to chemotherapy and others do not remains challenging. Identification of potential biomarkers for prediction of response is therefore of considerable interest. With recent progress in our understanding of the VEGF and EGFR pathways, we have also learned a great deal about potential molecular mechanisms underlying the clinical response to bevacizumab, cetuximab, and panitumumab. However, no clinically relevant predictors or prognostic markers have been identified.
Few studies have been published on potential biomarkers for efficacy and clinical outcome with regard to bevacizumab-based therapies. Dowlati reported that baseline VEGF serum levels in non–small-cell lung cancer patients were associated with overall survival in the E4599 trial.[43] Other groups have reported that germline polymorphisms of interleukin (IL)-8 and its receptors are associated with response and progression-free survival in refractory ovarian cancer patients treated with bevacizumab and low-dose cyclophosphamide.[44] More recently, single nucleotide polymorphisms (SNPs) in intercellular adhesion molecule (ICAM)-1 and G protein–coupled receptor of 78 kDa (GRP78) were significantly associated with response to FOLFOX/bevacizumab or XELOX/bevacizumab (unpublished results, Manegold P et al).
Limited data are available for combination studies of EGFR inhibitors and chemotherapy. Recently, numerous groups have reported that k-ras mutations are associated with resistance to EGFR inhibitors. Patients whose tumors do not have k-ras mutations are more likely to respond to cetuximab and panitumumab.[23,45-47]
In addition to the association with k-ras mutational status, the outcome of EGFR-inhibitor therapy is associated with skin rash. This acne-like effect is accompanied by an increased likelihood of response to cetuximab or panitumumab. Patients with grade 3/4 skin reactions showed the highest response rates with cetuximab treatment. Moreover, median survival appeared longer in these patients (9.1 vs 3.0 months).[32] However, it still remains unclear how skin manifestations should mirror the effects in the tumor.[18,32,48]
Two independent groups have shown that colorectal cancer patients with high gene-expression levels of the EGFR ligands epiregulin and amphiregulin are more likely to respond to cetuximab, suggesting an EGFR-dependent pathway in these tumors.[23,45]
Early data suggest that loss of PTEN expression is associated with nonresponse to cetuximab. Among 16 patients with normal expression of PTEN, 10 achieved a partial response (P < .001).[24] Though these preliminary results for molecular markers are encouraging, we need further evaluation of potential biomarkers and verification in large clinical trials.
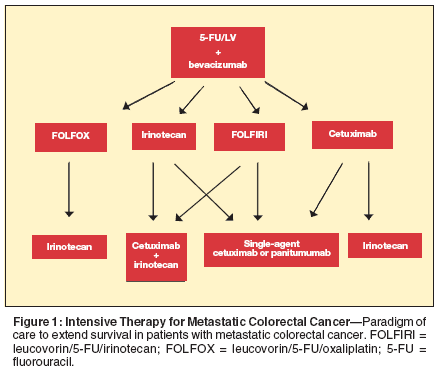
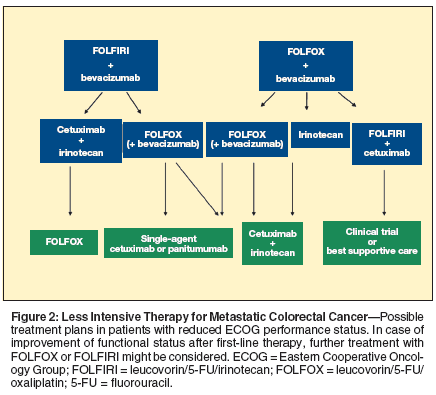
Paradigm of Care
With the introduction of targeted agents, we have achieved increased therapeutic efficacy and improved clinical outcomes in patients with metastatic colon cancer.[15,16,32,34-36,38,49-53] Moreover, the variety of targeted agents provides an opportunity to combine agents in an effort to improve antitumor activity.[18] Figures 1 and 2 demonstrate several options for incorporation of targeted agents in the clinic. However, several more options exist.
The addition of bevacizumab to 5-FU–based chemotherapeutic regimens has produced a clinically meaningful improvement in survival for advanced CRC patients.[15] FOLFOX and FOLFIRI have demonstrated similar response rates, time to progression, and overall survival in the first-line treatment of primarily unresectable metastatic disease. Preliminary data also suggest the safety and efficacy of these regimens in combination with bevacizumab; therefore, they should be considered as initial therapy for patients with good functional status.
The addition of EGFR inhibitors to the armamentarium has significantly increased the efficacy of irinotecan in second-line therapy. The EPIC trial[35] also showed an increased rate of R0 resections following cetuximab/irinotecan treatment in liver-limited mCRC. With increasing response rates, we have noticed increasing rates of curative resection.
The ultimate goal will be to tailor chemotherapy based on the patient’s molecular profile, not only to increase efficacy but also to decrease toxicity. Choices for initial and subsequent therapy also need to be evaluated based on toxicity profiles, ECOG status, treatment goal (ie, curative intent or not), and tumor-biology. Patients with the potential for cure should be treated aggressively and reevaluated by a surgeon at intervals. The balance of efficacy and toxicity is different for patients in a palliative setting.[16]
Conclusions
The treatment of metastatic colorectal cancer has substantially evolved during the past decade. Cetuximab and bevacizumab have proven their efficacy in the clinical setting, resulting in a doubling of survival time for mCRC patients. The efficacy of other agents such as vatalanib or sunitinib still need to be validated in this setting. However, the numerous therapeutic options create the challenge of developing optimized treatment strategies.
Biomarkers for predicting response to cetuximab and panitumumab have been investigated. The identification of predictive markers to antiangiogenic therapy is critical to our understanding of how best to use such treatment. Incorporation of these biomarkers into therapeutic strategies for mCRC will require validation in prospective clinical trials. This may lead to a more rational approach to the use of biologic as well as cytotoxic agents, with increased efficacy, minimized toxicity, and reduced treatment costs.
This article is reviewed here:
Building on the Foundation of 5-FU to Treat Metastatic Colorectal CancerSorting Out the Targeted-Agent Combinations for Colorectal Cancer
References:
References
1. Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2007. CA Cancer J Clin 57:43-66, 2007.
2. Ng K, Zhu AX: Targeting the epidermal growth factor receptor in metastatic colorectal cancer. Crit Rev Oncol Hematol 65:8-20, 2008.
3. Petrelli N, Douglass HO Jr, Herrera L, et al: The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: A prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J Clin Oncol 7:1419-1426, 1989.
4. Poon MA, O’Connell MJ, Moertel CG, et al: Biochemical modulation of fluorouracil: Evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol 7:1407-1418, 1989.
5. Piedbois P, Buyse M: What can we learn from a meta-analysis of trials testing the modulation of 5-FU by leucovorin? Advanced Colorectal Meta-analysis Project. Ann Oncol 4(suppl 2):15-9, 1993.
6. Capdevila J, Saura C, Macarulla T, et al: Monoclonal antibodies in the treatment of advanced colorectal cancer. Eur J Surg Oncol 33(suppl 2):S24-S34, 2007.
7. Folkman J: Tumor angiogenesis: Therapeutic implications. N Engl J Med 285:1182-1186, 1971.
8. Folkman J: Anti-angiogenesis: New concept for therapy of solid tumors. Ann Surg 175:409-416, 1972.
9. Longo R, Gasparini G: Challenges for patient selection with VEGF inhibitors. Cancer Chemother Pharmacol 60:151-170, 2007.
10. Shih T, Lindley C: Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther 28:1779-1802, 2006.
11. Dvorak HF: Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20:4368-4380, 2002.
12. Morabito A, De Maio E, Di Maio M, et al: Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: Current status and future directions. Oncologist 11:753-764, 2006.
13. Hasegawa Y, Takanashi S, Okudera K, et al: Vascular endothelial growth factor level as a prognostic determinant of small cell lung cancer in Japanese patients. Intern Med 44:26-34, 2005.
14. Wang TB, Deng MH, Qiu WS, et al: Association of serum vascular endothelial growth factor-C and lymphatic vessel density with lymph node metastasis and prognosis of patients with gastric cancer. World J Gastroenterol 13:1794-1798 (incl discussion), 2007.
15. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004.
16. Goldberg RM, Rothenberg ML, Van Cutsem E, et al: The continuum of care: A paradigm for the management of metastatic colorectal cancer. Oncologist 12:38-50, 2007.
17. Saltz L, Clarke S, Diaz-Rubio E, et al: Bevacizumab (Bev) in combination with XELOX or FOLFOX4: Updated efficacy results from XELOX-1/NO16966, a randomized phase III trial in first-line metastatic colorectal cancer (abstract 4028). J Clin Oncol 25(18S):170s, 2007.
18. Press MF, Lenz HJ: EGFR, HER2 and VEGF pathways: Validated targets for cancer treatment. Drugs 67:2045-2075, 2007.
19. Patard JJ, Pouessel D, Bensalah K, et al: Targeted therapy in renal cell carcinoma. World J Urol Feb 12, 2008 (epub ahead of print).
20. Saltz LB, Rosen LS, Marshall JL, et al: Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol 25:4793-4799, 2007.
21. Hecht JR, Trarbach T, Jaeger E, et al: A randomized, double-blind, placebo-controlled, phase III study in patients (Pts) with metastatic adenocarcinoma of the colon or rectum receiving first-line chemotherapy with oxaliplatin/5-fluorouracil/leucovorin and PTK787/ZK 222584 or placebo (CONFIRM-1) (abstract 3). J Clin Oncol 23(16S):2s, 2005.
22. Kohne C, Bajetta E, Lin E, et al: Final results of CONFIRM 2: A multinational, randomized, double-blind, phase III study in 2nd line patients (pts) with metastatic colorectal cancer (mCRC) receiving FOLFOX4 and PTK787/ZK 222584 (PTK/ZK) or placebo (abstract 4033). J Clin Oncol 25(18S):171s, 2007.
23. Khambata-Ford S, Garrett CR, Meropol NJ, et al: Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25:3230-3237, 2007.
24. Frattini M, Saletti P, Romagnani E, et al: PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer 97:1139-1145, 2007.
25. Adjei AA, Rowinsky EK: Novel anticancer agents in clinical development. Cancer Biol Ther 2:S5-S15, 2003.
26. Salomon DS, Brandt R, Ciardiello F, et al: Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19:183-232, 1995.
27. Syed S, Rowinsky E: The new generation of targeted therapies for breast cancer. Oncology (Williston Park) 17:1339-1356 (incl discussion), 2003.
28. Messa C, Russo F, Caruso MG, et al: EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol 37:285-289, 1998.
29. Porebska I, Harlozinska A, Bojarowski T: Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol 21:105-115, 2000.
30. Ciardiello F, Tortora G: A novel approach in the treatment of cancer: Targeting the epidermal growth factor receptor. Clin Cancer Res 7:2958-2970, 2001.
31. Tabernero J, Macarulla T, Ramos FJ, et al: Novel targeted therapies in the treatment of gastric and esophageal cancer. Ann Oncol 16:1740-1748, 2005.
32. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
33. Saltz LB, Meropol NJ, Loehrer PJ Sr, et al: Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201-1208, 2004.
34. Van Cutsem E, Nowacki M, Lang I, et al: Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): The CRYSTAL trial (abstract 4000). J Clin Oncol 25(18S):164s, 2007.
35. Sobrero AF, Fehrenbacher L, Rivera F, et al: Randomized phase III trial of cetuximab plus irinotecan versus irinotecan alone for metastatic colorectal cancer in 1298 patients who have failed prior oxaliplatin-based therapy: The EPIC trial (abstract LB-2). Program and abstracts of the American Association for Cancer Research Annual Meeting, Los Angeles, Apr 14-18, 2007.
36. Saltz LB, Lenz HJ, Kindler HL, et al: Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: The BOND-2 study. J Clin Oncol 25:4557-4561, 2007.
37. Hecht JR, Mitchell E, Chidiac T, et al: An updated analysis of safety and efficacy of oxaliplatin (Ox)/bevacizumab (bev) +/- panitumumab (pmab) for first-line treatment (tx) of metastatic colorectal cancer (mCRC) from a randomized, controlled trial (PACCE) (abstract 273). Gastrointestinal Cancers Symposium; Orlando, Fla; Jan 25-27, 2008. Available at www.asco.org/. Accessed March 7, 2008.
38. Hecht JR, Mitchell E, Chidiac T, et al: Interim results from PACCE: Irinotecan (Iri)/bevacizumab (bev) ± panitumumab (pmab) as first-line treatment (tx) for metastatic colorectal cancer (mCRC) (abstract 279). Gastrointestinal Cancers Symposium; Orlando, Fla; Jan 25-27, 2008. Available at www.asco.org/. Accessed March 7, 2008.
39. Zhang W, Gordon M, Schultheis AM, et al: FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 25:3712-3718, 2007.
40. Cascinu S, Berardi R, Salvagni S, et al: A combination of gefitinib and FOLFOX-4 as first-line treatment in advanced colorectal cancer patients. A GISCAD multicentre phase II study including a biological analysis of EGFR overexpression, amplification and NF-kB activation. Br J Cancer 98:71-76, 2008.
41. Vincent M, Kerr I, Dingle B, et al: Capecitabine (X) compared to X + erlotinib (E) as first-line treatment in patients (pts) with metastatic colorectal cancer (MCRC): Interim efficacy results from a randomized phase II study (abstract 14560). J Clin Oncol 25(18S):630s, 2007.
42. Tournigand C, Lledo G, Delord J, et al: Modified (m)FOLFOX7/bevacizumab (B) or modified (m)Xelox/bevacizumab with or without erlotinib (E) in first-line metastatic colorectal cancer (MCRC): Results of the feasibility phase of the DREAM-OPTIMOX3 study (GERCOR) (abstract 4097). J Clin Oncol 25(18S):187s, 2007.
43. Dowlati A, Gray R, Johnson DH, et al: Prospective correlative assessment of biomarkers in E4599 randomized phase II/III trial of carboplatin and paclitaxel ± bevacizumab in advanced non-small cell lung cancer (NSCLC) (abstract 7027). J Clin Oncol 24(18S):370s, 2006.
44. Schultheis AM, Yang D, Garcia AA, et al: Angiogenesis pathway gene polymorphisms associated with clinical outcome in recurrent ovarian cancer treated with low dose cyclophosphamide and bevacizumab: A California Consortium Trial (abstract 5017). J Clin Oncol 24(18S):260s, 2006.
45. Lievre A, Bachet JB, Boige V, et al: KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26:374-379, 2008.
46. Lievre A, Bachet JB, Le Corre D, et al: KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66:3992-3995, 2006.
47. Amado RG, Wolf M, Freeman D, et al: Analysis of KRAS mutations in patients with metastatic colorectal cancer receiving panitumumab monotherapy (abstract 0007). Presented at ECCO-14, the European Cancer Conference. Barcelona, Spain; Sept 23-27; 2007.
48. Saltz L, Kies M, Abbruzzese JL, et al: The presence and intensity of the cetuximab-induced acne-like rash predicts increased survival in studies across multiple malignancies (abstract 817). Proc Am Soc Clin Oncol 22:204, 2003.
49. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Colon cancer, v.1.2008. Available at www.nccn.org/. Accessed March 7, 2008.
50. Douillard JY, Cunningham D, Roth AD, et al: Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355:1041-1047, 2000.
51. Giantonio BJ, Catalano PJ, Meropol NJ, et al: Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539-1544, 2007.
52. Van Cutsem E, Peeters M, Siena S, et al: Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658-1664, 2007.
53. Van Cutsem E, Siena S, Humblet Y, et al: An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol 19:92-98, 2008.