The Radiation Oncology Perspective: Genomic vs Genetic Testing in Prostate Cancer
Decipher Prostate, Oncotype DX, and Prolaris are 3 gene expression testing options for patients with prostate cancers. Read as experts discuss them.


A Frontline Forum hosted by CancerNetwork during the 2025 American College of Radiation Oncology Summit in March focused on gene expression testing options for patients with prostate cancer.
The discussion centered on genetic vs genomic testing, current NCCN guidelines (Figure), and decisions based on which testing options to use. Following the program, CancerNetwork spoke with leading clinicians in the radiation oncology space regarding the use of these tests, androgen deprivation therapy (ADT), and the thought process for selecting patients for active surveillance.
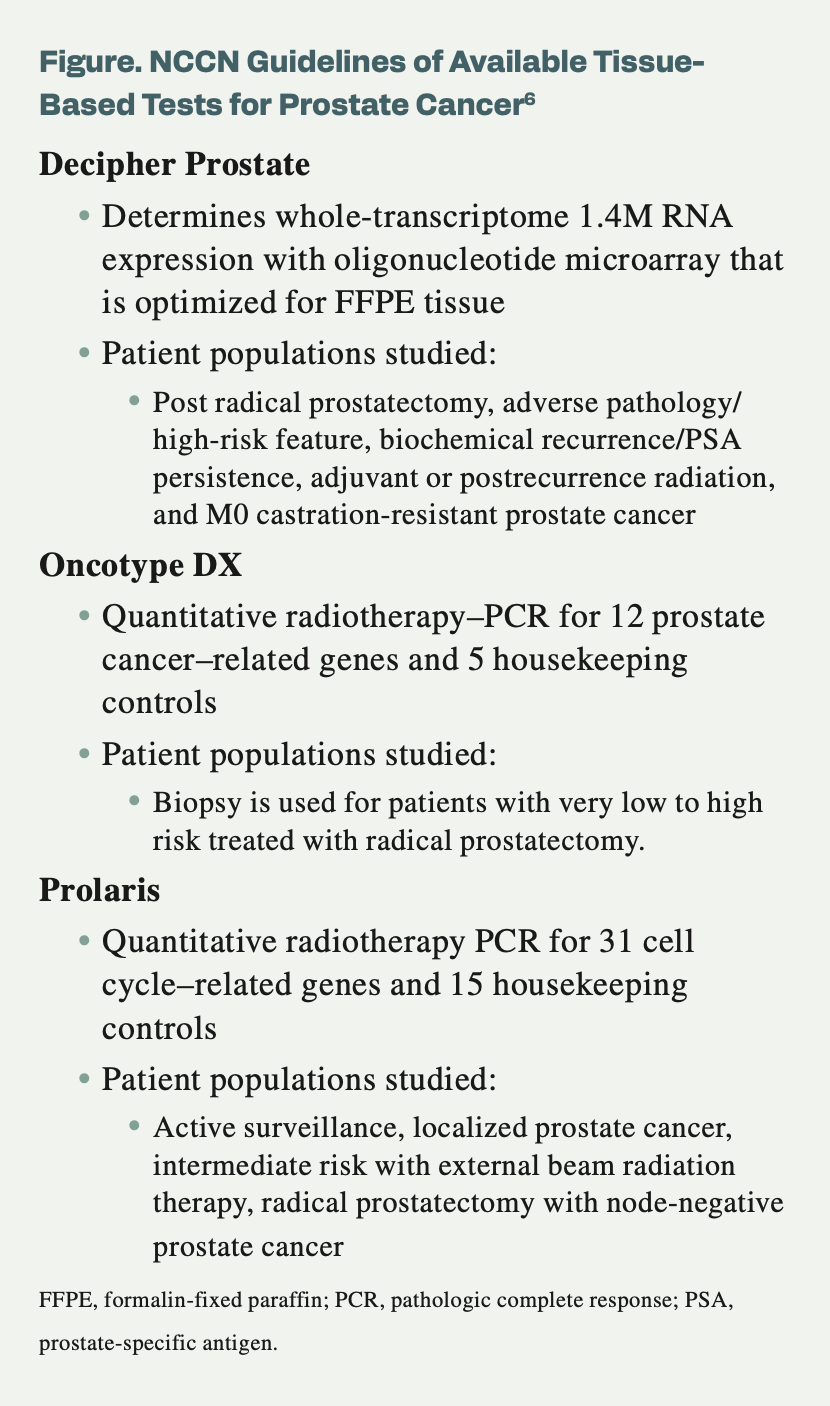
Figure. NCCN Guidelines of Available Tissue-Based Tests for Prostate Cancer6
To begin, the distinction between a genomic biomarker vs germline testing was mentioned. A genomic biomarker includes a measurable DNA or RNA characteristic that can be used to indicate normal biological processes, disease, or response to treatment. Germline testing is the analysis of DNA to identify genetic variations that may be associated with health risks or cancer predisposition.
Decipher Prostate has a level 1B evidence rating among the NCCN guidelines.1 A news release from the developer stated that Decipher received the rating because of evidence from the postbiopsy and postprostatectomy settings.
Decipher Prostate is a 22-gene test incorporating RNA whole-transcriptome analysis plus machine learning to help inform treatment decisions for patients with prostate cancer. Biopsy or surgically resected samples are used to provide a patient’s risk for developing metastases with standard treatment. This information will better personalize care as well as provide options that are less treatment-intensive. Validation for this test comes from more than 75 studies and more than 100,000 patients.
The Oncotype DX test is a 17-gene assay that was created to assess 12 cancer-related genes and 5 reference genes through biopsy tissue.2
A recent study looking into genomic classifiers across 10 studies reported the risk reclassification.3 In studies that had a low risk of bias and patients with prostate cancer who were very low or low risk, patients were more likely to have their risk levels classified as the same or lower with Oncotype DX at 100% to 88.1%, Decipher at 87.2% to 82.9%, and Prolaris at 76.9%. There was, however, 1 randomized trial of genomic classifiers with Oncotype DX that reclassified 34.5% of patients who were very low risk and 29.4% who were low risk to the high-risk category.
The study takeaway mentioned a need for more trials to better determine the role of genomic classifiers for patients with newly diagnosed prostate cancer who are considering first-line treatment.3
The NCCN guidelines include Prolaris in the prostate cancer guidelines with category level 2A of evidence.5 This is a molecular diagnostic test that can provide personalized information regarding the aggressiveness of a tumor and can determine whether a patient should pursue treatment. Of note, a news release from Myriad Genetics stated that it is the only biomarker test to quantify the benefits of ADT to radiation therapy.4
Findings from a study by Tward et al found the combined clinical cell-cycle risk (CCR) score for a single therapy that is a continuous variable was able to prognosticate metastases (HR, 3.97; 95% CI, 2.61-6.06) as well as for the dichotomized threshold (HR, 15.90; 95% CI, 5.43-46.52).5 If patients were given single-modality therapy of radiation therapy or surgery, the 10-year Kaplan Meier score was 4.3% (95% CI, 1.0%-17.1%) for those with a CCR score below the threshold and 20.4% (95% CI, 13.2%-30.7%) for those with a score above the threshold.
The authors stated that if the CCR score was below the 2.112 multimodality threshold, patients could safely avoid multimodality therapy. Additionally, clinicians can use the CCR scores to counsel patients on which type of therapy would be most effective for intermediate or high-risk prostate cancer.
A discussion during the program also occurred around clinical decision-making. The program also focused on active surveillance, which is used to identify appropriate candidates who are eligible for this treatment with favorable or intermediate disease; treatment modality selection included guiding choices between single and multimodality approaches; and ADT optimization was determining if there was a benefit by adding ADT to radiation therapy.
Read on to see key highlights from genitourinary radiation oncologists and their takes on genomic/genetic testing.
Q / What is your comprehensive approach to selecting patients for active surveillance, particularly how genomic testing has refined this process beyond traditional clinical parameters for favorable intermediate-risk disease?
Kim / When we think about patients for active surveillance, we first start with the traditional clinical PATH variable, so we restratify them per the NCCN [guidelines]. That’s where most of our data have been generated, and that’s where our discussion begins. In our practice, it’s changed as we’ve integrated genomic testing in all these patients, so all our patients receive a genomic test, and we find it to be an important data point as we engage in shared decision-making about active surveillance vs treatment. For our patients [with] favorable intermediate [risk] who often get the Prolaris genomic test, we try to use that in conjunction with the risk groups to first make a decision of whether a patient is appropriate for active surveillance. Sometimes, surprisingly, with a genomic test, we find that even though they look like a traditional active surveillance candidate, they are not, due to their genomic score.
Q / When Prolaris results indicate a patient may be appropriate for active surveillance but conventional clinical factors suggest otherwise, how do you reconcile this discordance? What threshold in genomic testing gives you confidence to recommend surveillance?
Kim / This is an increasing issue as we are using more and more genomic testing. I first try to remember how our big trials were done because that gives us the best data. I tell the patient that the genomic test is prognostic but not predictive, so it doesn’t tell us which treatment we should or should not do, although we hope that certainly [will be the case] in the future. It gives us another important data point to engage in shared decision-making around the risks and benefits of, say, active surveillance. There are risks and benefits of adding hormone therapy through radiation, and so that’s where we find a lot of value, and so do our patients.
Q / What is a memorable case where genomic testing significantly altered your surveillance recommendation, highlighting how the data informed your clinical judgment beyond what would have been possible with conventional risk stratification alone?
Kim / I recently had a 59-year-old patient with traditional NCCN, favorable intermediate risk, 3 + 4 disease, and 2 of 12 cores. Without genomic testing, he wanted surveillance. He didn’t want treatment. We got a Prolaris score, and unfortunately, it did come back as a higher risk than we would have thought. He went from traditional active surveillance to being considered for multimodality therapy. That prompted further testing, such as a fusion biopsy, which ended up upgrading his Gleason score, and he ended up going on active treatment with hormone therapy and radiation.
Q / How has genomic biomarker testing fundamentally transformed the prostate cancer treatment paradigm over the past decade, particularly regarding the shift from population-based to precision medicine approaches?
Lee / In comparison with breast cancer, prostate cancer is behind the curve concerning the frequency of ordering genomic and germline testing. It’s exciting to be a part of this change in practice patterns, where now we can offer a more customized approach for patients and more patient-centric treatment decision-making through biomarker testing for eachpatient. When I’m [treating] patients, I like to evaluate their clinical factors, and their comorbidities, but also have the additional information that’s gained by biomarker testing and their germline testing results. This helps us to get a more complete picture of their risks and helps us to give them information specific to their clinical situation so that the patient and I both have greater peace of mind when they’re making decisions to move forward with these important treatments for their cancer.
Q / Based on your experience, what framework would you recommend for institutions implementing comprehensive genomic and genetic testing programs, and what key barriers have you observed that hindered successful adoption?
Lee / In the past, ordering biomarkers and germline testing was not the standard for radiation oncologists. In this modern era, as the landscape is changing, it’s becoming very key to what we do and making those crucial clinical decisions with individual patients. As new institutions are implementing these new steps and adding the results of these tests to their discussions with patients, they’ll find that it helps to facilitate those discussions and helps them to have a more in-depth and patient-centered discussion with their patients on how these tests can be beneficial and help them gain peace of mind and move forward with the specific treatment that they choose.
Q / In your view, what are the most compelling validation studies supporting genomic biomarker use in clinical practice, and how should clinicians evaluate the growing body of evidence to determine which tests offer actionable information vs those that may not yet justify routine implementation?
Lee / As we’ve implemented genomic and germline testing at our institution over the years, I’ve found it very beneficial to patients. I found that referring providers and clinicians whom we work with very closely are also now frequently ordering these tests before the patient gets to me, as we work as teams with urologists and other medical oncologists who specialize in prostate cancer by having genomic testing and germline testing results. It helps us work better as a team to provide the best-customized care for our individual patients.
Q / Having evaluated the major genomic biomarker platforms, what distinguishes Prolaris in terms of actionable clinical information, particularly regarding its ability to quantify the absolute benefit of adding ADT to radiation therapy?
Finkelstein / Biomarker testing is extremely important in today’s world of prostate cancer. One of the tests that led the field was Prolaris. Prolaris was built upon a series of data that matured over time and a set of papers by Jonathan D. Tward, MD, PhD, FASTRO. One of the first things they did was validate a specific clinical cell-cycle risk [CCR] score threshold that identified patients with intermediate- or high-risk prostate cancer who may safely admit or require multimodal therapy, thereby tailoring treatment intensity, building upon that foundation. These were all exciting things that brought new, next-generation genomics to what we standardly did in conventional staging.
Q / In what specific clinical scenarios have you found Prolaris to be most practice-changing compared with other available tests, and how has this influenced your institutional approach to genomic testing implementation?
Finkelstein / There’s been incredible pieces of data with respect to biomarkers. When we use Prolaris as a model system, we can see that they’ve influenced how we approach active surveillance and how we approach the ability to potentially take away the need for ADT in our patients. These 2 pieces are most valuable for radiation oncologists.
Q / Based on extensive clinical experience with Prolaris, what would you identify as the most compelling evidence supporting its use that clinicians should understand when selecting genomic tests for patients with prostate cancer?
Finkelstein / As a radiation oncologist, we build with our wonderful colleagues a way to try to figure out how best to individualize and personalize care. Tward said it best when we want to use the right tool for the right patient at the right time. There are very extensive data supporting the use of Prolaris as a model system for patients with active surveillance. Additionally, there’s a strong set of data about potentially the ability to omit ADT in patients who might be receiving radiation therapy. These 2 are the most important when radiation oncologists see patients in their clinics.
References
- Veracyte’s Decipher Prostate test receives highest evidence-level rating among molecular tests in updated prostate cancer NCCN guidelines. News release. Veracyte. February 27, 2024. Accessed April 3, 2025. https://tinyurl.com/2v8yd7tb
- Janes JL, Boyer MJ, Bennett JP, et al. The 17-gene genomic prostate score test is prognostic for outcomes after primary external beam radiation therapy in men with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2023;115(1):120-131. doi:10.1016/j.ijrobp.2022.06.101
- Tabriz AA, Boyer MJ, Gordon AM, et al. Impact of genomic classifiers on risk stratification and treatment intensity in patients with localized prostate cancer : a systematic review. Ann Intern Med. 2025;178(2):218-228. doi:10.7326/ANNALS-24-00700
- NCCN prostate cancer guidelines reinforce status of Myriad Genetics’ Prolaris test as an 'advanced tool' recommended for prognostic assessment. News release. Myriad genetics. December 9, 2024. Accessed April 3, 2025. https://tinyurl.com/ms8kazwr
- Tward JD, Schlomm T, Bardot S, et al. Personalizing localized prostate cancer: validation of a combined clinical cell-cycle risk (CCR) score threshold for prognosticating benefit from multimodality therapy. Clin Genitourin Cancer. 2021;19(4):296-304.e3. doi:10.1016/j.clgc.2021.01.003
- NCCN Clinical Practice Guidelines in Oncology. Prostate cancer; version 1.2025. Accessed April 3, 2025. https://tinyurl.com/24vyz9nr
