Introduction
Systemic therapy for renal cell carcinoma (RCC) has rapidly evolved over the past 5 years. Currently, there are 11 US Food and Drug Administration–approved systemic therapies for RCC. Since 2015 alone, three new second-line therapies-nivolumab,[1] cabozantinib,[2] and lenvatinib with everolimus[3]-have been approved, with data showing improvement in overall survival. Effectively, the field has tested agents for metastatic disease in only two clinical settings: primary management of metastatic disease (first-line) and after progression with a first-line therapy (second-line; typically a vascular endothelial growth factor receptor [VEGFR] inhibitor); however, there are no category 1 data that support the use of any agent in the third-line setting.
From experience and anecdote, most physicians recognize that the available agents have activity in the third line. Thus, physicians have made therapeutic decisions based on extrapolations of efficacy and toxicity from the second-line setting into the third line and beyond. Since patients with kidney cancer are living longer, it is important to consider how to optimally use the available and emerging treatments for this disease to extend survival and optimize quality of life.
Moving Beyond Lines of Therapy: Use of Molecular Classifications
Despite the growing insight into the biology of RCC that has fueled the explosion of therapeutics, the classification of this dynamic disease has been disappointingly static. Although it remains accurate and helpful to classify kidney cancers by histologic subtypes, it is well recognized that there is a tremendous amount of molecular heterogeneity in this disease that exists between patients (inter-patient), between metastatic tumors (inter-tumoral), and even between cells within a given tumor (intra-tumoral).[4] It is clear that “truncal” mutational events, such as those related to the von Hippel-Lindau pathway, have identified broadly active therapies (eg, those active on hypoxia-inducible factor/VEGFR–driven signaling). Nonetheless, since heterogeneity is thought to drive a large part of resistance to therapy, and given the number of biologic pathways leading to resistance, it is unlikely that a single pathway disruption accounts for resistance. In the current treatment paradigm, VEGFR inhibition is typically used as primary treatment. Until the introduction of nivolumab, repeated and increasingly forceful inhibition of VEGFR was a mainstay of therapy. Checkpoint inhibitors have now emerged as an effective and often preferred second-line treatment, if for no other reason than to provide a holiday from adverse events related to VEGFR inhibitor therapy and/or to delay expected toxicities from VEGFR and/or mammalian target of rapamycin (mTOR) inhibition.
Although checkpoint inhibitors and other immunomodulatory approaches in part address issues of heterogeneity by virtue of the plasticity of the immune response, they provide dramatic effects in a subset of patients and little to no benefit for others.
The move toward molecular classification has identified alternative molecular targets, such as BRAF, MET, TSC1/2, and others, which have facilitated treatment selection in the third-line setting and beyond. What is becoming increasingly clear, however, is that historic profiles, such as those from nephrectomy specimens, may not reflect the contemporary status of a previously treated tumor that has evolved under the selective pressure of medical therapy. Tissue biopsies remain the gold standard for reclassification of the tumor. Serial biopsies are not always possible due to anatomic location or declining performance status. Thus, there remains a need to gain molecular insights.
Blood-based genomics have arisen as a potential solution to this problem. The use of circulating tumor cells and cell-free DNA have shown value in multiple models, including prostate,[5,6] lung,[7] colon,[8] and breast cancers.[9,10] There is a growing movement to use blood-based testing to identify and monitor therapeutic targets for patients undergoing therapy with signal transduction inhibitors. Work with circulating tumor cells shows that they may be useful as a tissue surrogate even for highly sensitive molecular characterizations, such as next-generation sequencing.[11]
At the current time, however, there is an absence of reliable markers for response to immunotherapy, whether this refers to more classical therapies, such as high-dose interleukin-2, or contemporary approaches, such as programmed death 1 (PD-1) inhibition. Although there has been sustained interest in the use of programmed death ligand 1 (PD-L1) expression as an immunohistochemical marker, it is less clear whether expression in the tumor or the immune microenvironment is more important. In addition, it is still clear that in RCC and other cancers, the absence of PD-L1 staining does not preclude benefit from checkpoint inhibitor therapy.
Given the expanding range of therapeutic approaches, the use of molecular classification of tissue may prove to be highly useful to oncologists when treating their patients. To date, while these studies have not neatly stratified patients into responders and nonresponders, the use of targeted therapies generally increases the likelihood of a patient responding to a particular class of agents. This paradigm (of genomic preselection) has become an increasingly common practice among researchers, such that this thinking has moved from retrospective analyses of phase III studies, to stratification in phase III, phase II, and even phase I studies. Although seemingly restrictive in some ways, this approach meets the growing demand for more effective agents and the urgent need for personalization of care in oncology.
Value-Based Decision Making in Kidney Cancer
Although efficacy and toxicity in a relatively amorphous manner have been used by oncologists to match patient to therapy in the first-, second-, and even third-line settings, the increasing number of options and limited number of comparative studies have created a need to identify other factors that impact the appropriateness of various therapies.
Although historically it was considered taboo to discuss cost in the setting of advanced cancer, it is important to recognize the soaring costs of oncology care that impact kidney cancer patients and their healthcare providers. It is no secret that the cost of the agents used to treat metastatic RCC has skyrocketed due to the resources required by a pharmaceutical company to develop safe, effective, and approved drugs. Although oncologists have continued to attach the greatest value to efficacy of therapy and toxicities associated with therapy, it is now becoming more important for physicians and patients to understand comparative benefit, which does include cost as a parameter.
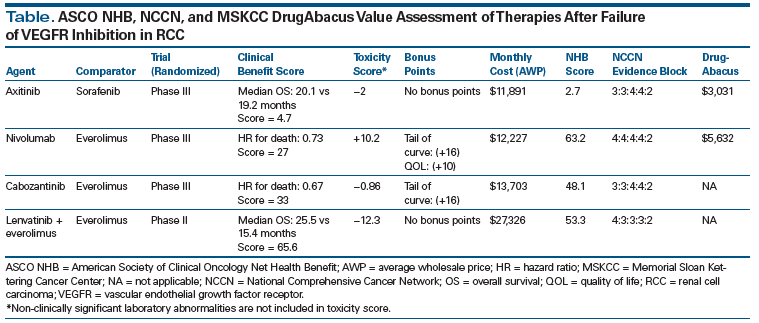
Over the past few years, several organizations have put forward structured frameworks that provide a means for physicians to compare the relative benefit of active therapies based on available clinical trial data. These value-based tools include the American Society of Clinical Oncology (ASCO) Net Health Benefit (NHB) score, the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS), the National Comprehensive Cancer Network (NCCN) Framework, and the Memorial Sloan Kettering Cancer Center (MSKCC) DrugAbacus.
KEY POINTS
- There are no agents
with comparative
efficacy and toxicity
data in the third-line
kidney cancer setting. - Molecular classifications
done by tissue or
blood characterization
can be helpful in refining
options. - Clinical trials in this
setting remain an
important part of
contemporary care. - Comparative value
should be considered
as part of therapeutic
decisions in kidney
cancer in the third-line
setting and beyond.
Each framework attempts to standardize the available clinical data into a point- or rank-based system that assigns value to agents based on associated clinical activity, toxicity, and financial burden. Examples of this evaluation in kidney cancer are provided in the Table, using the ASCO NHB, NCCN, and MSKCC DrugAbacus systems. Using the ASCO NHB system, higher scores indicate a higher net-benefit value. The NCCN system ranks treatments on a 5-point scale in terms of efficacy, safety, quality of evidence, consistency of evidence, and affordability as determined by an expert panel, where higher scores indicate greater value. Finally, the MSKCC DrugAbacus system creates a dollar value for each therapy based on a number of benefit and toxicity factors, and this allows for comparison of the value of therapy with actual drug cost. Using this system, the value of an ideal treatment is closer to its actual price. Using such methodologies, physicians have additional information to aid in decision making. At this time, the ESMO-MCBS forms are available online (http://www.esmo.org/Policy/Magnitude-of-Clinical-Benefit-Scale/Scale-Evaluation-Forms), as is the MSKCC DrugAbacus (www.drugabacus.org/drug-abacus/tool/). The ASCO NHB tool will be available as both a web-based tool and smartphone app in the near future.
Conclusions
Changes in the landscape of RCC therapy have brought new challenges in caring for patients. The third-line setting has historically been where new agents are tested for activity to identify those worthy of further development in the second or first line; however, there remains an urgent need to support clinical studies in the third line. It is here that the field continues to grow, and therefore we strongly recommend that practitioners seek out trials for their patients even before the second line of therapy, when appropriate. Given the number of trials and agents, the use of molecular tools must become part of the practicing oncologist’s standard behavior. As these tools (including blood-based diagnostics) are refined and continue to further decrease in price, we will finally move beyond the historic lines of therapy into truly personalized care in kidney cancer, and oncology in general.
As we draw closer to this goal of personalized care in kidney cancer, practical considerations can aid in decision making. In 2016, these must include consideration of cost, as well as benefit. Given the value-based tools produced and vetted by leading professional and academic organizations, oncologists now have the ability to make more informed comparisons when making treatment recommendations to patients. Ultimately, with a combination of molecular tools, valuation of care, and good clinical practice, the care of patients with advanced kidney cancer will become an example of optimized personalized oncology care in the near future.
Financial Disclosure:Dr. Posadas has served on an advisory board for Pfizer; he has also received travel reimbursement from Tracon Pharma for presenting to a scientific advisory board.
References:
1. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803-13.
2. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814-23.
3. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473-82.
4. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883-92.
5. Scher HI, Heller G, Molina A, et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J Clin Oncol. 2015;33:1348-55.
6. Chen JF, Ho H, Lichterman J, et al. Subclassification of prostate cancer circulating tumor cells by nuclear size reveals very small nuclear circulating tumor cells in patients with visceral metastases. Cancer. 2015;121:3240-51.
7. Yanagita M, Redig AJ, Paweletz CP, et al. A prospective evaluation of circulating tumor cells and cell-free DNA in EGFR mutant non-small cell lung cancer patients treated with erlotinib on a phase II trial. Clin Cancer Res. 2016 Jun 8. [Epub ahead of print]
8. Lu CY, Tsai HL, Uen YH, et al. Circulating tumor cells as a surrogate marker for determining clinical outcome to mFOLFOX chemotherapy in patients with stage III colon cancer. Br J Cancer. 2013;108:791-7.
9. Shaw JA, Guttery DS, Hills A, et al. Mutation analysis of cell-free DNA and single circulating tumor cells in metastatic breast cancer patients with high CTC counts. Clin Cancer Res. 2016 Jun 22. [Epub ahead of print]
10. Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7:302ra133.
11. Zhao L, Lu YT, Li F, et al. High-purity prostate circulating tumor cell isolation by a polymer nanofiber-embedded microchip for whole exome sequencing. Adv Mater. 2013;25:2897-902.