Thromboembolic Complications of Malignancy: Part 2
Thromboembolism affects many patients with solid tumors and clonalhematologic malignancies. Thromboprophylaxis with low-molecularweightheparin (LMWH) is indicated for surgery and other high-risksituations, but not routinely for central venous catheters or nonsurgical,ambulatory management. Thrombotic events require full anticoagulationfor the duration of active disease and/or the prothromboticstimulus. LMWHs are safe and more effective than both unfractionatedheparin for initial therapy and warfarin for secondary prevention. Antiinflammatoryand antiangiogenic properties might account for thisadvantage and for a survival benefit of chronic LMWH in subgroupsof cancer patients. Ongoing studies are characterizing the cost-effectivenessand antitumor mechanisms of LMWHs, the potential utility ofnewer anticoagulants, and the ability of predictive models to identifyhigh-risk candidates for thromboprophylaxis.
Thromboembolism affects many patients with solid tumors and clonal hematologic malignancies. Thromboprophylaxis with low-molecularweight heparin (LMWH) is indicated for surgery and other high-risk situations, but not routinely for central venous catheters or nonsurgical, ambulatory management. Thrombotic events require full anticoagulation for the duration of active disease and/or the prothrombotic stimulus. LMWHs are safe and more effective than both unfractionated heparin for initial therapy and warfarin for secondary prevention. Antiinflammatory and antiangiogenic properties might account for this advantage and for a survival benefit of chronic LMWH in subgroups of cancer patients. Ongoing studies are characterizing the cost-effectiveness and antitumor mechanisms of LMWHs, the potential utility of newer anticoagulants, and the ability of predictive models to identify high-risk candidates for thromboprophylaxis.
As we noted in the June issue of ONCOLOGY, recent or active cancer is a powerful prothrombotic risk factor, greatly increasing the possibility of venous thromboembolism (VTE). Part 1 of this review considered the mechanisms involved in thromboembolic complications, their clinical presentation, and the many prothrombotic risks that have been identified in patients with solid tumors and clonal hematologic malignancies and disorders. In part 2, we will address prophylaxis and treatment of VTE in this setting. VTE Prophylaxis in Patients With MalignancyPrimary Surgical Prophylaxis
The significant incidence of VTE in patients undergoing surgical procedures has led to the development of graded recommendations for prophylaxis in these populations based on assessment of underlying risk.[1] Current recommendations from the American College of Chest Physicians (ACCP) suggest that patients with malignancy should receive prophylaxis according to their high state of risk (Table 1). However, the presence of malignancy may influence the selection of specific pharmacologic agents for VTE prophylaxis, the dosing of these agents, and the duration of therapy. In patients undergoing general surgery, unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) are equally effective in preventing postoperative thrombosis, according to a meta-analysis of trials comparing both therapies, without regard to dose or duration of therapy.[2] Some of the trials evaluated in this meta-analysis included patients with malignancy, and these patients were compared to patients without cancer in a subanalysis. Bleeding and thrombosis occurred more frequently in patients undergoing cancer surgery vs noncancer surgery, but UFH and LMWH were equally effective and safe in both populations. Several trials provide additional details regarding the appropriate dosing and duration of thromboprophylaxis in surgical patients with malignancy. In patients undergoing surgery for gynecologic malignancy, UFH at 5,000 U given three times daily was more effective than UFH at 5,000 U given twice daily, without an increase in bleeding complications.[3] In the Enoxacan Study, 1,115 cancer patients undergoing abdominal or pelvic surgery were randomized to prophylaxis with enoxaparin (Lovenox) at 40 mg daily vs UFH at 5,000 U three times daily.[4] No differences were found in the rate of venographically evident VTE at 3 months (14.7% vs 18.2%), or in the rate of total bleeding complications (18.7% vs 17.1%). In patients with malignancy undergoing general surgery and randomized to dalteparin (Fragmin) at 5,000 U vs 2,500 U daily, the higher dose was more effective in preventing deep venous thrombosis detected by fibrinogen uptake (8.5% vs 14%; P < .001), without an increased risk of bleeding (4.6% vs 3.6%; NS).[5]
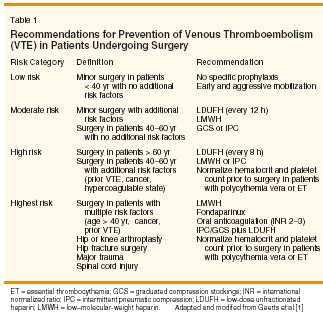
Although VTE prophylaxis is typically continued until hospital discharge, the risk of VTE extends beyond the hospital stay. Extended prophylaxis was investigated in the Enoxacan II Study of 322 patients undergoing surgery for abdominal or pelvic cancer.[6] After initial prophylaxis with enoxaparin, 40 mg daily for 6 to 10 days, patients were randomized to continue placebo or enoxaparin for an additional 3 weeks. Extended prophylaxis was associated with a 60% reduction in the incidence of total VTE at 1 month (4.8% vs 12%; P = .02) and at 3 months (5.5% vs 13.8%; P = .01) without a significant increase in the risk of minor, major, or total bleeding. Thus, once-daily LMWH and threetimes- daily UFH appear to be equally effective in preventing VTE in patients with malignancy undergoing general surgery. Similar findings have been observed in patients undergoing surgery for breast and gynecologic malignancies.[ 7] Despite higher drug acquisition cost, LMWH offers advantages over UFH, including once-daily dosing, which improves patient acceptance and limits nursing time, and a lower incidence of heparin-induced thrombocytopenia. Decision analytic models have estimated that LMWH is cost-effective for prophylaxis in gynecologic oncology surgery.[8] However, similar analyses have not yet compared once-daily LMWH vs threetimes- daily UFH in other settings, nor are there data regarding the cost implications of extended prophylaxis. Well-designed prospective trials are needed to gather this information for optimal, evidence-based practice. Similar aggressive prophylactic anticoagulation strategies are indicated for the perioperative management of patients with hematologic malignancies, including polycythemia vera and essential thrombocythemia. In patients with polycythemia vera, the hematocrit should be normalized before elective surgery (ie, to < 45% in men and < 42% in women) to minimize the risks of thrombosis and bleeding. It is also recommended that the platelet count be normalized preoperatively in polycythemia vera or essential thrombocythemia patients with high-risk features (ie, age > 60 years or prior thromboembolic event). Although lowdose aspirin (ie, 100 mg/d) minimizes the nonsurgical risk of arterial and venous thromboemboli in patients with polycythemia vera,[9] the benefit of aspirin in the perioperative setting is untested and one must weigh the theoretical benefit against the potential risks of bleeding. Primary Prophylaxis in Hospitalized Medically Ill Patients
The role of thromboprophylaxis in immobile or bedridden hospitalized and medically ill patients is well established (reviewed in [1]). A number of clinical trials showed that either twice-daily or three-times-daily dosing of UFH effectively reduces the incidence of VTE in this population. A meta-analysis of the general surgical literature suggests that three-times-daily UFH is more effective for VTE prophylaxis than twicedaily dosing[10]; however, no trials comparing these regimens have been conducted in acutely ill medical patients. More recent clinical trials have established the effectiveness of enoxaparin, 40 mg daily, or dalteparin, 5,000 U daily, compared to placebo, and of fondaparinux (Arixtra), 2.5 mg daily. Two studies comparing UFH, 5,000 U three times daily, with enoxaparin, 40 mg daily, in acutely ill patients, including those with malignancy, found similar rates of VTE as detected by either duplex ultrasonography or venography. As risk status increased, so did the overall effectiveness of enoxaparin compared to UFH, although this was not specifical- ly evaluated in patients with malignancy. A subanalysis of the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial comparing enoxaparin to placebo in acutely ill medical patients found that the most significant risk factors for thrombosis in this population were age > 75 years, history of VTE, and malignancy.[11] Although it is clear that thromboprophylaxis is effective and safe in bedbound medically ill patients, several controversies have yet to be resolved in patients with malignancy, including: (1) the relative efficacy of three-timesdaily UFH vs LMWH; (2) the role of chemotherapy- or radiation-induced thrombocytopenia in bleeding risk; (3) the role of ambulatory status on VTE risk; and (4) the appropriate duration of therapy. Although enoxaparin has been determined to be more cost-effective for VTE prophylaxis in acutely ill medical patients than twice-daily UFH,[12] no pharmacoeconomic studies have yet compared enoxaparin to three-times-daily UFH, nor have patients with malignancy been specifically addressed. Prophylaxis in Ambulatory Patients
Low-intensity oral anticoagulation with warfarin was observed to prevent VTE during chemotherapy in 311 patients with stage IV breast cancer.[13] Those patients were randomized to placebo or to fixed-dose warfarin, 1 mg daily for 6 weeks, followed by dosing adjustments to maintain the international normalized ratio (INR) between 1.3 and 1.9. During 6 months of therapy, anticoagulation was associated with an 85% reduction in the relative risk of VTE without a significant increase in bleeding complications or a significant increase in costs.[14] By comparison, a more recent study in patients with limited-stage small cell lung cancer treated with chemotherapy and radiation found that oral prophylactic anticoagulation had no influence on survival.[15] Similarly, the Fragmin Advanced Malignancy Outcome Study (FAMOUS) trial comparing dalteparin, 5,000 U daily, to placebo for a mean of 9 months in ambulatory patients with a variety of different advanced malignancies found no difference in the rate of VTE or overall survival between the two cohorts.[16] Therefore, among patients with solid tumors, thromboprophylaxis should be reserved for those who are hospitalized and acutely ill, those undergoing surgical procedures, and those with other individual high-risk features (Table 2).
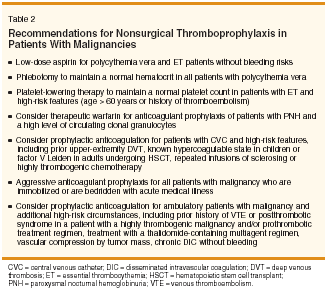
Prophylactic therapy for patients with polycythemia vera include phlebotomy to maintain the hematocrit in the normal range (ie, < 45% for men and < 42% for women)[17] and daily low-dose aspirin (81 to 100 mg) for those who are not at increased risk of bleeding (Table 2).[9] Cytoreductive therapy should be given to normalize the platelet count in patients with polycythemia vera or essential thrombocythemia who also have high-risk features, including prior thromboembolism or age > 60 years; and considerations given for patients with comorbid cardiovascular risks (eg, smoking, diabetes, atherosclerotic disease).[17,18] Hydroxyurea is the cytoreductive agent of choice for patients with myeloproliferative disorders, based on the limited available information from randomized clinical trials.[18,19] Anagrelide is inferior to hydroxyurea for patients with essential thrombocythemia,[ 19] but it is a reasonable second-line option. Interferon alfa should be used in women of childbearing potential to avoid the teratogenic effects of the other agents. Primary prophylaxis with therapeutic warfarin has been recommended for patients with paroxysmal nocturnal hemoglobinuria (PNH) who have a high level of circulating PNH granulocytes[ 20]; however, this intervention must be weighed against the risks of bleeding, especially in patients with severe hemolysis and thrombocytopenia. Prophylaxis for Indwelling Central Venous Catheters
Clinical trials evaluating anticoagulation for prevention of central venous catheter (CVC) thrombosis have produced conflicting results (reviewed in [21,22]). Oral anticoagulation with "minidose" warfarin, at 1 mg daily, reduced CVC clot formation by roughly fourfold (9.5% compared to 37.5% for no treatment) in 84 patients with cancer. By contrast, a more recent study found no benefit among 100 cancer patients treated with warfarin, 1 mg daily, compared to placebo. The potential hemorrhag ic risk of prophylaxis must also be considered. Roughly one-third of patients receiving minidose warfarin with continuous-infusion 5-FU reached an INR > 1.5, and 23% of those anticoagulated patients suffered a bleeding complication.[23] Similar discrepant outcomes have been observed with LMWH prophylaxis. Dalteparin has been found to be both effective and ineffective in preventing clot formation associated with CVCs.[22] Because of these conflicting results, neither fixed, lowdose warfarin nor LMWH are currently recommended for routine prevention of CVC thrombosis in patients with malignancy.[1] However, prophylaxis with adjusted-dose warfarin or LMWH should be considered on a case-by-case basis for patients who are felt to be at a high risk of line-associated thrombosis (Table 2). Treatment of Venous ThromboembolismInitial Treatment
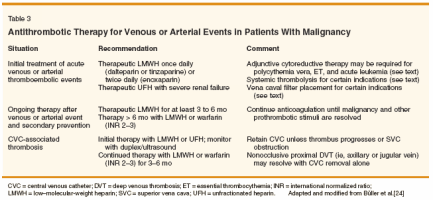
Current evidence-based guidelines for the initial treatment of VTE recommend that patients receive concurrent warfarin and either UFH or LMWH for a minimum of 5 days and until the INR is stable and ≥ 2 (Table 3).[24] Meta-analyses of a number of clinical trials comparing UFH and LMWH in the initial treatment of VTE concluded that these agents are equally safe and effective, with similar rates of recurrent VTE and major bleeding. However, LMWHs are associated with a 24% to 29% reduction in the risk of mortality compared to UFH. In patients with cancer, this mortality benefit may be magnified. In the subgroup of 279 patients with cancer included in one meta-analysis, mortality was 25.9% among patients treated with UFH and 16.7% in patients treated with LMWH.[25] In addition, the combined results of two early trials comparing UFH and LMWH in the initial treatment of VTE found a mortality rate of 31% among 67 cancer patients treated with UFH, compared to 11% in 62 cancer patients treated with LMWH (P < .005).[26] Although UFH and LMWH have not been compared in a head-to-head trial in patients with cancer, these subgroup analyses of comparative trials strongly support the contention that LMWH provides a mortality benefit over UFH for the initial treatment of VTE in cancer patients. When enoxaparin is selected as the LMWH for initial treatment of VTE, the typical strategy is 1 mg/kg of total body weight every 12 hours. This schedule was compared to 1.5 mg/kg daily and to UFH in 900 patients with acute VTE.[27] The incidence of recurrent VTE at 3 months was similar for both once- and twice-daily enoxaparin in all patients (4.4% vs 2.9%), but in the subgroup of 96 patients with malignancy, once-daily dosing was inferior (12.2% vs 6.4%). In comparison, once-daily dosing of tinzaparin (Innohep) at 175 U/kg and dalteparin at 200 U/kg are effective as initial treatments of VTE in patients with malignancy.[ 28,29] In patients with thrombocytopenia (eg, due to myelosuppressive chemotherapy or hematopoietic failure), the platelet count must be monitored and transfusions given to maintain the count at ≥ 50,000/μL. In addition, patients on UFH or LMWH should be monitored for development of heparin-induced thrombocytopenia. For CVC-associated events, fibrin sheath occlusion is usually cleared by instillation of a fibrinolytic agent, such as urokinase (Abbokinase) or tissuetype plasminogen activator (tPA). This procedure can be safely carried out in thrombocytopenic patients. Nonocclusive subclavian clots and proximal CVC-associated deep venous thromboses are usually managed with therapeutic anticoagulation (either UFH or LMWH) without removal of the catheter.[30] Prospective monitoring with duplex ultrasound must be performed to ensure resolution of the clot. Extension of proximal thromboses on therapeutic anticoagulation or more extensive/occlusive central deep venous thromboses (eg, with superior vena cava syndrome) require catheter removal followed by full anticoagulation and, in some cases, thrombolysis. Full anticoagulation, with LMWH or warfarin, should be continued for at least 3 to 6 months, and subsequent prophylactic anticoagulation should be given for long-term indwelling catheters or if a new CVC is placed. Acute thromboembolic events in patients with myeloma, leukemias, polycythemia vera, essential thrombocythemia, or PNH are managed initially with therapeutic LMWH or UFH, similar to events in patients with solid tumors. Thrombocytopenic patients should be closely monitored and transfused with platelets as indicated. Additional considerations include the possible need for urgent white cell reduction (ie, by leukapheresis) in patients with acute myeloid leukemia and leukostasis (usually with circulating blast counts greater than 50,000 to 100,000/μL), urgent red cell reduction (ie, by erythrocytapheresis) in patients with polycythemia vera and uncontrolled erythrocytosis, and urgent platelet reduction (ie, by plateletpheresis) in patients with polycythemia vera or essential thrombocythemia and uncontrolled thrombocytosis. Systemically administered thrombolysis should be considered for selected patients with massive ileofemoral deep venous thrombosis who are at risk for limb gangrene (eg, with phlegmasia alba dolens) and patients with pulmonary embolism who are hemodynamically unstable.[24] Local administration of thrombolytic agents is not recommended. Vena caval interruption, most commonly accomplished by placement of a wire filter, is indicated for patients with acute deep venous thrombosis and/or pulmonary embolism with absolute contraindications to anticoagulation or with bleeding or recurrent pulmonary embolism while on appropriate anticoagulant therapy. Permanent and removable wire filters can be placed in either the inferior or superior vena cava using fluoroscopic or ultrasound guidance. These devices reduce the rate of recurrent pulmonary embolism, but they are associated with an increased 2-year risk of recurrent deep venous thrombosis. Therefore, full anticoagulation should be resumed as soon as possible in patients with chronic filters.[24]
Ongoing Treatment
Following the initial treatment of VTE in general medical and surgical patients, an oral vitamin K antagonist is typically continued for at least 3 months in order to prevent recurrence.[ 24] However, oral anticoagulation is less successful in patients with cancer. An inception cohort study evaluated the long-term outcomes of 842 consecutive patients with VTE, 181 of whom had known cancer.[31] All patients were treated initially with either UFH or LMWH and subsequently switched to oral anticoagulant therapy dosed to maintain the INR between 2 and 3 for 3 to 6 months (notably, 56% of patients with malignancy continued oral anticoagulation for longer than 6 months). The 12- month cumulative incidence of recurrent VTE was 20.7% in the cancer patients, compared to only 6.8% in patients without malignancy (hazard ratio [HR]: 3.2; 95% confidence interval [CI]: 1.9-5.4). Major bleeding occurred in 12.4% of patients with malignancy, compared to only 4.9% of patients without malignancy (HR: 2.2; 95% CI: 1.2-4.1). Other studies of cancer patients whose vitamin K antagonist therapy was managed in dedicated anticoagulation clinics have found similar significantly high rates of recurrent thrombosis and major bleeding compared to patients without cancer, as well as high rates of warfarin-related emergency room visits and hospital admissions.[32] Several clinical trials have compared oral anticoagulation to LMWH for the long-term management of VTE in patients with malignancy. In a small open-label study, 138 cancer patients with VTE were randomized to receive enoxaparin, 1.5 mg/kg once daily, or enoxaparin followed by warfarin dosed to an INR of 2 to 3.[33] After 3 months of therapy, patients who received enoxaparin for the full treatment course had a trend toward fewer major bleeding events (7.5% vs 16.9%; P = .09) and a lower mortality rate (11.3% vs 22.7%; P = .07) compared to patients who received warfarin. Unfortunately, this trial was underpowered to reveal statistically significant differences in these outcomes. In addition, the number of symptomatic, objectively confirmed recurrent thromboembolic events (2 vs 3 events, respectively) were too infrequent to see a difference between the two treatment groups. Nonetheless, it suggested a benefit of LMWH over warfarin in the long-term treatment of VTE in patients with malignancy. The Comparison of Low-molecular- weight heparin vs Oral anticoagulant Therapy (CLOT) study provides the most substantive evidence of the benefits of LMWH over warfarin for the continued management of VTE in patients with cancer.[29] In this trial, 672 patients with malignancy and new onset VTE were randomized to dalteparin, 200 U/kg once daily for a month, followed by 150 U/kg once daily for 5 months, or to dalteparin, 200 U/kg once daily for 5 to 7 days, with concurrent acenocoumarol or warfarin, dosed to an INR of 2 to 3, and continuation of the coumarin derivative alone for a total of 6 months. Symptomatic, recurrent VTE occurred in 9% of patients treated with long-term dalteparin compared to 17% treated with an oral anticoagulant (P = .002). This study did not find a difference in major bleeding (6% vs 4%) nor in 6-month mortality (39% vs 41%) for the two treatment groups, respectively. However, a post hoc analysis revealed a significant difference in 12-month mortality among patients with nonmetastatic disease treated with dalteparin vs coumarin (20% vs 36%; HR: 0.50; 95% CI: 0.27- 0.95; P = .03).[34] These studies indicate that LMWHs are superior to warfarin in protecting against recurrent events and improving survival after cancer-associated thrombosis. As a possible explanation, there is growing evidence that at least some LMWHs have anti-inflammatory and antiangiogenic properties that are not provided by warfarin or UFH.[35] On a practical level, LMWHs are easier to manage because dosing is based on weight, laboratory monitoring is not required, and the short half-life facilitates adjustments during thrombocytopenic periods. LMWHs can also be safely interrupted for invasive procedures or surgery without the need for complicated "bridging" protocols. Based on recent evidence, the current ACCP guidelines recommend that patients with cancer-associated thrombosis receive therapeutic LMWH for 3 to 6 months (Table 3).[24] It remains to be seen how quickly clinical practice patterns will change toward uniform use of long-term LMWHs for secondary prevention of VTE. The cost of LMWHs may be a deterrent for health-care plans that limit coverage and reimbursement for drug expenses. In addition, it is unknown whether LMWHs are superior to warfarin for long-term treatment of CVCassociated thrombosis or for secondary prevention in patients with hematologic malignancies, such as myeloma, lymphomas, essential thrombocythemia, polycythemia vera, or PNH. A recent international survey of nearly 4,000 physicians who treat cancer patients found that LMWH was the most common choice for the initial treatment of VTE, but oral anticoagulation was favored as long-term treatment in the setting of malignancy.[36] Duration of Therapy
Chronic anticoagulant therapy beyond 3 and 6 months has not been systematically evaluated in patients with malignancy-associated VTE, nor has LMWH been compared to oral anticoagulation for longer than the first 6 months. However, chronic anticoagulation is superior to shorter-term therapy among patients with idiopathic thrombosis, including many who have hypercoagulable conditions that are persistent risk factors for thrombosis.[ 24] Also, the rate of recurrent VTE in patients with malignancy is high despite therapeutic anticoagulation.[31] Thus, malignancy should be considered a persistent strong risk factor for thrombosis, and post-VTE treatment should continue for as long as the neoplastic disorder and/or the interventionrelated prothrombotic risks are present. For these reasons, the current ACCP guidelines recommend that either LMWH or oral anticoagulation be continued indefinitely, or until the malignancy resolves, after the patient has received LMWH for 3 to 6 months (Table 3).[24] Further investigation is warranted to clarify the details of treatment duration in subgroups of patients with malignancy-associated events. Cost Considerations
For most patients with idiopathic or predisposed VTE, initial therapy with LMWHs provides a significant cost savings compared to UFH because LMWHs can be used on an outpatient basis and outcomes with the two agents, including the rates of recurrent VTE and bleeding, are equivalent (reviewed in [37]). By comparison, most patients with malignancy-associated VTE require initial treatment in the hospital because of disease-associated comorbidities. Despite the need for inpatient management, a cost-minimization model, which assumes equal efficacy for UFH and LMWH, determined that initial LMWH therapy is still cost-effective for cancer-associated VTE, primarily because length of stay is decreased and less anticoagulant monitoring is required.[38] These observations, combined with the data suggesting that initial LMWH reduces mortality, strongly favor LMWHs over UFH for primary management of VTE in patients with malignancies. The long-term use of LMWHs for cancer-associated VTE has significant economic implications because of the high drug acquisition costs compared to vitamin K antagonists. One pharmacoeconomic analysis addressed the impact of chronic LMWH among general VTE patients treated on randomized clinical trials.[39] LMWH was deemed cost-effective, compared to warfarin, when a cost per qualityadjusted life years model was applied. However, prophylactic doses of LMWHs were used in those randomized trials (eg, enoxaparin at 40 mg/d); therefore, they cannot be extended to the therapeutic doses of LMWH required for patients with cancer. Rigorous economic analyses comparing long-term LMWHs with warfarin for patients with malignancy have not been published. However, simple calculations can estimate the theoretical costs in this setting. For example, the estimated cost of VTE in cancer patients, inflated for 2002 US dollars, is $20,065 per episode.[ 40] The outcomes data from the CLOT study suggest that 8 out of every 100 patients with cancer-associated VTE treated with LMWH will develop a recurrent VTE, compared to 16 out of 100 treated with warfarin.[ 29] Thus, the projected treatment costs associated with these episodes would be $160,520 for the LMWH group and $321,040 for the warfarin group. The average wholesale prices for dalteparin for an 80-kg patient receiving doses as per the CLOT study are approximately $80 per day for the first month (at 200 U/kg/d) and $60 per day for the following 5 months (at 150 U/kg/d). The overall cost of therapeutic dalteparin for 100 patients treated for 6 months is, therefore, $1,140,000. By comparison, the average wholesale price for warfarin, when dosed at 5 mg/d, is $26.80 per month; which translates into $16,080 for 6 months of treatment for 100 patients. Based on drug costs alone, a 6-month course of dalteparin exceeds the cost of warfarin (dosed at 5-10 mg/d) by approximately $1.12 million. The cost savings related to fewer secondary VTE events on LMWH (ie, $160,520) would lower this difference between dalteparin and warfarin to $963,400 (ie, $9,634 per patient for a 6-month treatment course). For the costs of long-term dalteparin to equal long-term warfarin, significant additional warfarin-related expenses would have to accrue. These might include monitoring costs (ie, for prothrombin time/INR levels [up to $36 per test], travel expenses, and time away from work), physician/pharmacist charges, expenses for treatment of minor bleeding complications, and other costs related to quality of life. Clearly, a full pharmacoeconomic analysis needs to be conducted to determine the cost implications of long-term LMWHs in patients with malignancy. Future Perspectives The results of ongoing studies should significantly expand our current knowledge of the pathogenetic mechanisms, hemostatic mediators, and clinical laboratory markers important in malignancy- associated thrombosis. Validated laboratory and clinical predictive models will hopefully facilitate our ability to identify high-risk patients who would benefit from prophylactic interventions. Studies in animal models are characterizing the antithrombotic, antiinflammatory, and antitumor activities of LMWHs and these observations should guide their optimal use in selected patient populations. Very little is known about the potential utility of approved anti-Xa agents and direct thrombin inhibitors in patients with malignancy-associated VTE. In addition, a number of experimental anticoagulants are in various stages of development, and these may offer unique advantages over currently approved drugs.[41] Ximelagatran, an oral direct thrombin inhibitor, is under investigation as an alternative to warfarin. It offers a consistent antithrombotic effect at fixed doses without the need for routine coagulation monitoring and without concerns related to drug-drug, drug-food, and drug-disease state interactions.[ 42] Additional oral direct thrombin inhibitors are under study, as are a number of oral and injectable indirect inhibitors of factor Xa. Given the role of tissue factor as a major thrombogenic mediator in many patients with cancer, investigational agents that directly inhibit tissue factor and factor VIIa (including recombinant tissue factor pathway inhibitor and a recombinant polypeptide analog of a canine hookworm protein [NAPc2][41]) merit evaluation for applications in tumor-associated thrombosis. Whether the distinct pharmacology of these agents, or their pharmacokinetic and pharmacodynamic properties, will translate into any meaningful role in the management of thrombosis in malignancy will ultimately need to be determined in well-designed clinical trials.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Geerts WH, Pineo GF, Heit JA, et al: Prevention of venous thromboembolism. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:338S-400S, 2004.
2. Mismetti P, Laporte S, Darmon JY, et al: Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 88:913-930, 2001.
3. Clarke-Pearson DL, DeLong E, Synam IS, et al: A controlled trial of two low-dose heparin regimens for the prevention of post-operative deep vein thrombosis. Obstet Gynecol 75:684- 689, 1990.
4. ENOXACAN Study Group: Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: A double-blind, randomized multicenter trial with venographic assessment. Br J Surg 84:1099-1103, 1997.
5. Bergqvist D, Burmark US, Flordal PA, et al: Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 vs 5000 XaI units in 2070 patients. Br J Surg 82:496-501, 1995.
6. Bergqvist D, Agnelli G, Cohen A, et al: Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 346:975-980, 2002.
7. Heilmann L, von Tempelhoff GF, Kirkpatrick C, et al: Comparison of unfractionated heparin versus low molecular weight heparin for deep vein thrombosis prophylaxis during breast and pelvic cancer surgery. Efficacy, safety and follow-up. Clin Appl Thromb Hemost 4:268-273, 1998.
8. Dainty L, Maxwell GL, Clarke-Pearson DL, et al: Cost-effectiveness of combination thromboembolism prophylaxis in gynecologic oncology surgery. Gynecol Oncol 93:366-373, 2004.
9. Landolfi R, Marchioli R, Kutti J, et al: Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 350:114-124, 2004.
10. Clagett GP, Reisch JS: Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg 208:227-240, 1988.
11. Alikhan R, Cohen AT, Combe S, et al: Risk factors for venous thromboembolism in hospitalized patients with acute medical illness. Analysis of the MEDENOX Study. Arch Intern Med 164:963-968, 2004.
12. McGarry LJ, Thompson D, Weinstein MC, et al: Cost effectiveness of thromboprophylaxis with a low-molecular-weight heparin vs unfractionated heparin in acutely ill medical inpatients. Am J Manag Care 10:632-642, 2004.
13. Levine M, Hirsh J, Gent M, et al: Doubleblind, randomized trial of very low dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet 343:886-889, 1994.
14. Rajan R, Gafni A, Levine M, et al: Very low dose warfarin prophylaxis to prevent thromboembolism in women with metastatic breast cancer receiving chemotherapy: An economic evaluation. J Clin Oncol 13:42-46, 1995.
15. Maurer LH, Herdon JE, Hollis DR, et al: Randomized trial of chemotherapy and radiation with or without warfarin for limited-stage small cell lung cancer. J Clin Oncol 15:3378-3387, 1997.
16. Kakkar A, Levine M, Kadziola Z, et al: Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: The fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol 22:1944-1948, 2004.
17. Spivak JL, Barosi G, Tognoni G, et al: Chronic myeloproliferative disorders. Hematology (Am Soc Hematol Educ Program):200-224, 2003.
18. Cortelazzo S, Finazzi G, Ruggeri M, et al: Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 332:1132–1136, 1995.
19. Green A, Campbell P, Buck G, et al: The Medical Research Council PT1 trial in essential thrombocythemia (abstract 6). Blood 104:5a, 2004.
20. Hall C, Richards S, Hillmen P: Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood 102:3587-3591, 2003.
21. Kuter D: Thrombotic complications of central venous catheters in cancer patients. Oncologist 9:207-216, 2004.
22. Lopez JA, Kearon C, Lee AY: Deep venous thrombosis. Hematology (Am Soc Hematol Educ Program) 439-456, 2004.
23. Masci G, Magagnoli M, Zucali PA, et al: Minidose warfarin prophylaxis for catheter-associated thrombosis in cancer patients: Can it be safely associated with fluorouracil-based chemotherapy? J Clin Oncol 21:736-739, 2003.
24. Büller HR, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:401S-428S, 2004.
25. Gould MK, Dembitzer AD, Doyle RL, et al: Low molecular weight heparins compared with unfractionated heparin for the treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med 130:800-809, 1999.
26. Green D, Hull RD, Brant R, et al: Lower mortality in cancer patients treated with low molecular weight heparin versus standard heparin. Lancet 339:1476, 1992.
27. Merli G, Spiro TE, Olsson CG, et al: Subcutaneous enoxaparin once or twice daily compared to intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med 134:191-202, 2001.
28. Hull RD, Pineo GF, Mah AF, et al: A randomized trial evaluating long-term low-molecular- weight heparin therapy for three months vs. intravenous heparin followed by warfarin sodium in patients with current cancer (abstract). Thromb Haemost 90:P137a, 2003.
29. Lee AY, Levine MN, Baker RI, et al: Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 349:146-153, 2003.
30. Verso M, Agnelli G: Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 21:3665-3675, 2003.
31. Prandoni P, Lensing AW, Piccioli A, et al: Recurrent venous thromboembolism and bleeding complication during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 100:3484-3488, 2002.
32. Wittkowsky AK, Veenstra D, Blough D: Clinical outcomes and medical resource utilization in patients with malignancy treated with warfarin (abstract). J Thromb Haemost 1:P1373, 2003.
33. Meyer G, Marjanovic Z, Valcke J, et al: Comparison of low molecular weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer. Arch Intern Med 162:1729-1735, 2002.
34. Lee AY, Rickles FR, Julian JA, et al: Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol 23:2123-2129, 2005.
35. Hoppensteadt D, Walenga JM, Fareed J, et al: Heparin, low-molecular-weight heparins, and heparin pentasaccharide: Basic and clinical differentiation. Hematol Oncol Clin N Am 17:313-341, 2003.
36. Kakkar AK, Levine M, Pinedo HM, et al: Venous thrombosis in cancer patients: Insights from the FRONTLINE survey. Oncologist 8:381- 388, 2003.
37. Segal JB, Bolger DT, Jenckes MW, et al: Outpatient therapy with low molecular weight heparin for the treatment of venous thromboembolism: A review of efficacy, safety, and costs. Am J Med 115:298-308, 2003.
38. Avritscher EB, Cantor SB, Shih YCT, et al: Cost-minimization analysis of low molecular weight heparin (dalteparin) compared with unfractionated heparin for inpatient treatment of cancer patients with deep vein thrombosis. Support Care Cancer 12:531-536, 2004.
39. Marchetti M, Pistorio A, Barone M, et al: Low molecular weight heparin versus warfarin for secondary prophylaxis of venous thromboembolism: A cost-effectiveness analysis. Am J Med 111:130-139, 2001.
40. Elting L, Escalante C, Cooksley C, et al: Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med 164:1653-1661, 2004.
41. Weitz JI, Hirsh J, Samama MM: New anticoagulant drugs. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:265S-286S, 2004.
42. Nutescu E, Wittkowsky AK: Direct thrombin inhibitors for anticoagulation. Ann Pharmacother 38:99-109, 2004.
43. Aujesky D, Smith KJ, Cornuz J, et al: Costeffectiveness of low-molecular-weight heparin for secondary prophylaxis of cancer-related venous thromboembolism. Thromb Haemost 93:592-595, 2005.
Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.