Commentary (Stinchcombe et al): Perspectives on Salvage Therapy for Non–Small-Cell Lung Cancer
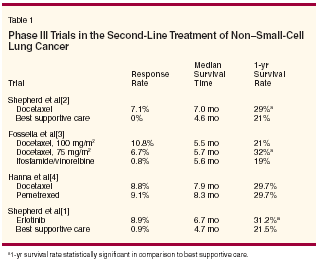
We applaud Dr. Cappuzzo andcolleagues for an excellentreview of an emerging fieldin lung cancer treatment. Since 2000,three drugs (docetaxel [Taxotere],pemetrexed [Alimta], and erlotinib[Tarceva]) have been approved by theUS Food and Drug Administration(FDA) for second-line therapy in non–small-cell lung cancer (NSCLC) basedon the results of phase III trials (seeTable 1).[1-4] It is also possible thatsimilar approval will be sought for otherdrugs (eg, topotecan [Hycamtin]),[5]and gefitinib (Iressa) remains an optionfor treatment in the third-line setting.
We applaud Dr. Cappuzzo and colleagues for an excellent review of an emerging field in lung cancer treatment. Since 2000, three drugs (docetaxel [Taxotere], pemetrexed [Alimta], and erlotinib [Tarceva]) have been approved by the US Food and Drug Administration (FDA) for second-line therapy in non- small-cell lung cancer (NSCLC) based on the results of phase III trials (see Table 1).[1-4] It is also possible that similar approval will be sought for other drugs (eg, topotecan [Hycamtin]),[5] and gefitinib (Iressa) remains an option for treatment in the third-line setting. Docetaxel Trials
Docetaxel is currently approved at a dose and schedule of 75 mg/m2 every 21 days, and the primary toxicity is myelosuppression. Three phase III trials in the second-line setting have compared a weekly treatment schedule to the standard every-3-week schedule. The first trial, by Camps et al, compared standard docetaxel to docetaxel at 36 mg/m2 weekly for 6 consecutive weeks in an 8-week course. This trial found a higher rate of anemia (86.4% vs 72.5% P = .027), diarrhea (30.7% vs 12.1% P = .003), and epigastric pain (20.5% vs 8.8% P = .034) in the weekly-treatment arm. The every-3-week arm had a higher incidence of alopecia (60.4% vs 40.9% P = .011).[6] The time to tumor progression (2.7 vs 2.9 months, P = NS) and the 1-year survival rate (29.2% vs 21.8%, P = NS) were similar; however, there was a significant difference in median survival, favoring the every-3-week schedule (7.5 vs 5.4 months, P = .04).[7] The second phase III trial was performed by Schuette et al, who compared standard docetaxel every 3 weeks to docetaxel at 35 mg/m2 weekly for 3 weeks followed by a week without treatment (one cycle = 28 days).[8] This study found fewer grade 3/4 toxicities with weekly treatment (P = .001), and significantly less grade 3/4 anemia, neutropenia, and alopecia. The rate of febrile neutropenia was similar (2.0 % vs 1.0%), as were overall response rates (9.7% vs 7.6%). Median survival time on the every-3- week arm was 5.8 months, but median survival had not been reached on the weekly arm at the time of the 2004 annual meeting of the American Society of Clinical Oncology (ASCO). Interestingly, patients who had not received prior paclitaxel therapy had a significantly longer survival with weekly treatment (P = .03). The third trial compared standard docetaxel to docetaxel at 33.3 mg/m2 weekly for 6 weeks every 8 weeks (one cycle = 8 weeks).[9] The every- 3-week schedule produced more neutropenia, febrile neutropenia, and alopecia, as well as a higher rate of grade 3/4 hematologic toxicity. The weekly arm was associated with more diarrhea. The response rate (2.7% vs 5.5%), median survival time (29 vs 25 weeks), and 1-year survival rate (21% vs 31%) were similar in the every-3-week arm and weekly arm, respectively. These trials may provide information about potentially increased efficacy in some subsets of patients such as those who are taxane-naive or have poor performance status and may be less able to tolerate myelosuppression. Our impression of these trials is that the weekly strategy with docetaxel does not offer major advantages over the conventional every-3-week schedule. Novel Agents
A new class of drugs-the epidermal growth factor receptor oral tyrosine kinase inhibitors (EGFR TKIs)-have recently been developed and evaluated in phase III trials as well. The results of the BR.21 trial indicated a superior survival to treatment with erlotinib over best supportive care.[1] It should be noted that the BR.21 trial allowed the enrollment of patients who had received only one prior regimen (second-line patients). This issue is pertinent in that the National Cancer Institute of Canada had previously established docetaxel as the standard treatment in the second-line setting. Presumably, patients entered onto BR.21 were not suitable for docetaxel therapy. Ongoing phase III trials are comparing the cytotoxic approach (docetaxel or pemetrexed) to therapy with an oral EGFR TKI (gefitinib or erlotinib) in the second-line setting. (Docetaxel was compared to gefitinib in a randomized phase II trial reported at the 2005 ASCO meeting, showing similar rates of response, survival, and symptom relief [the primary endpoint].[ 10]) A toxicity advantage for gefitinib was reported in this trial. Until the results from these very important trials are known, we believe the cytotoxic approach in the secondline setting remains optimal for most patients. However, this is a debated issue; treatment options should be discussed with the patient and made on an individual basis.
Cautionary Notes
As Dr. Cappuzzo and colleagues note, clinical, pathologic, and molecular markers have been reported that putatively identify subsets of patients more likely to respond to oral EGFR TKI therapy. Examples of all three categories are reviewed in the text, with the major focus being on mutations of the EGFR. Although we are encouraged by progress in deciphering the determinants of disease response to this novel drug class, a note of caution needs to be sounded. At the present time, none of the reported markers is mature enough for broad clinical implementation. Interpretation of even the wellstudied EGFR mutation may be more complex than initially thought. We provide a single cautionary example to serve as a model for the many types of errors that might be made. In reports by Lynch[11] and others, there is good evidence that although mutant tumors respond with higher frequency to EGFR TKIs, they may respond more vigorously to conventional treatments as well (as presented at ASCO 2005). Therefore, attempts to shuttle patients with mutated EGFR away from conventional cytotoxic chemotherapy in favor of EGFR TKIs may in fact be denying them the most effective therapies. In individual cases, there may be reasonable arguments to direct therapeutic decisions based on current riskfactor knowledge, but as a general rule, we prefer to offer therapy based on the evidence provided by clinical trials. The Iressa Survival Evaluation in Lung Cancer (ISEL) trial failed to reveal a survival benefit of gefitinib therapy over best supportive care in the second-line setting.[12] The fact that this trial did not reveal a survival benefit for EGFR TKI therapy, in contrast to the results of BR.21 trial, is perplexing. There may have been clinically relevant differences in the patient populations or in the percentage of patients who were refractory to prior treatment. The gefitinib dose used in this trial (250 mg daily) is approximately one-third of the maximum tolerated dose,[13] whereas patients on the BR.21 trial received erlotinib at the maximum tolerated dose (150 mg daily). This may have been a contributing factor to different results between the two trials. We await the full publication of results of this trial, as well as the results of the phase III trial comparing every-3-week docetaxel to gefitinib at 250 mg daily in the second- line setting before making a final determination on the efficacy of gefitinib at this dose level. Other Agents
Pemetrexed every 3 weeks has similar efficacy and causes less myelosuppression than every-3-week docetaxel.[4] The favorable toxicity profile of this agent and the convenient schedule make it an appealing choice in this situation. The dose of 500 mg/m2 was determined in patients before the relationship between vitamin supplementation and toxicity had been determined. The maximum tolerated dose of this agent in patients on vitamin supplementation is currently being explored.[14] Whether there could be increased efficacy at a higher dose would have to be explored in clinical trials. The other agents that have been explored in this situation are gemcitabine (Gemzar) and weekly paclitaxel. Promising phase II data of these agents are available[15-17]; however, phase III data evaluating the efficacy and toxicity of these agents are currently not available. These two agents have an attractive toxicity profile and are reasonable alternatives when patients are not candidates for an FDA-approved agent or are intolerant of FDA-approved agents. Two other agents that may have a role in the second-line setting are cetuximab (Erbitux) and bevacizumab (Avastin). In a phase II trial in the second-line setting, single-agent cetuximab demonstrated a 3.3% response rate, and 28% of patients had stable disease.[18] In another phase II trial in the second-line setting, the combination of cetuximab and docetaxel had a promising response rate of 28%, and 17% of patients had stable disease.[19] Promising results from a phase II trial with the combination of erlotinib and bevacizumab[ 20] as well as phase II trials of cetuximab will have to be further evaluated in phase III investigations before they can be considered as standard of care. Given the recent results of Eastern Cooperative Oncology Group (ECOG) 4599,[21] the integration of bevacizumab into second- line therapy may be complicated by the fact that more patients will be receiving bevacizumab therapy in the first-line setting. Conclusions
In summary, the paradigm of second- line (and later) therapy providing benefit to previously treated patients with advanced NSCLC has been established in randomized phase III trials, and available options have increased as a result of these trials. The standard remains single-agent therapy, although further research is needed to define subsets of patients who may benefit from more aggressive two-drug approaches (ie, prolonged progression-free interval, improved quality of life, etc). Likewise, we need to define subsets of patients who may benefit from cytotoxic vs EGFR TKI- based therapy. The optimal duration of therapy in the second-line setting and the integration of new therapeutics (eg, bevacizumab, cetuximab, and others) promise to be areas of active investigation as well. Ongoing and soon-to-be-initiated clinical trials promise to provide more information about this rapidly evolving field. We must be thoughtful in the design of future trials and ask questions based on our understanding of the biology of lung cancer, also taking into consideration the heterogeneity of patients with this disease.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Shepherd FA, Pereira TE, Ciuleanu EH, et al: A randomized placebo-controlled trial of erlotinib in patients with advanced non-small cell lung cancer (NSCLC) following failure of 1st or 2nd line chemotherapy. A National Institute of Canada Clinical Trials (NCIC CTG) trial (abstract 7022). Proceedings of the 2004 Annual Meeting of the American Society of Clinical Oncology. Available at www.asco.org. Accessed June 13, 2005.
2. Shepherd FA, Dancey J, Ramlau R, et al: Prospective randomized trial of docetaxel versus best supportive care in patients with nonsmall- cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18:2095-2103, 2000.
3. Fossella FV, DeVore R, Kerr RN, et al: Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 18:2354- 2362, 2000.
4. Hanna NH, Shepherd FA, Rosell R, et al: A phase III study of pemetrexed vs docetaxel in patients with recurrent non-small cell lung cancer (NSCLC) who were previously treated with chemotherapy (abstract 2503). Proc Am Soc Clin Oncol 22:622, 2003.
5. Ramlau R, Gervais R, Krzakowski M, et al: Oral topotecan demonstrates clinical activity in relapsed non-small cell lung cancer. Results of an open-label, phase III study (387) comparing oral topotecan to intravenous docetaxel (abstract 7017). J Clin Oncol 23(16S):625s, 2005.
6. Camps C, Massuti B, Jimenez AM, et al: Second-line docetaxel administrated every 3 weeks versus weekly in advanced non-small cell lung cancer (NSCLC): A Spanish Lung Cancer Group (SLCG) phase III trial (abstract 2514). Proc Am Soc Clin Oncol 22:625, 2003.
7. Evans TL: Highlights from the Tenth World Conference on Lung Cancer. Oncologist 9:232-238, 2004.
8. Schuette W, Nagel S, Serke M, et al: Second- line chemotherapy for advanced non-small cell lung cancer (NSCLC) with weekly versus three-weekly docetaxel: Results of a randomized phase III study (abstract 7036). Proc Am Soc Clin Oncol 23:622, 2004.
9. Gridelli C, Gallo C, Di Maio M, et al: A randomised clinical trial of two docetaxel regimens (weekly vs 3 week) in the second-line treatment of non-small-cell lung cancer. The DISTAL 01 study. Br J Cancer 91:1996-2004, 2004.
10. Cufer T, Vrdoljak E: Results from a phase II, open label, randomized study (SIGN) comparing gefitinib with docetaxel as second-line therapy in patients with advanced (stage IIIB or IV) non-small cell lung cancer (abstract 7035). J Clin Oncol 23(16S):629s, 2005.
11. Lynch TJ, Bell D, Haber D, et al: Correlation of molecular markers including mutations with clinical outcomes in advanced non-small cell lung cancer (NSCLC) patients (pts) treated with gefitinib, chemotherapy, or cehmotherapy and gefitinib in IDEAL and INTACT (abstract 7006). J Clin Oncol 23(16S):622s, 2005.
12. Thatcher N, Chang A, Purvish P, et al: Results of a phase III placebo controlled study (ISEL) of gefitinib (IRESSA) plus best supportive care (BSC) in patients with advanced nonsmall cell lung cancer (NSCLC) who had received 1 or 2 prior chemotherapy regimens (abstract LB-6). Proc Am Assoc Cancer Res vol 46, 2005.
13. Negoro S, Nakagawa K, Fukuoka M, et al: Final results of a phase I intermittant doseescalation of ZD1839 (‘Iressa’) in Japanese patients with various solid tumors (abstract 1292). Proc Am Soc Clin Oncol 20:324A, 2001.
14. Lee JS, Hsia TC, Yang CH, et al: Intrapatient dose escalation to 800 mg/m2 pemetrexed is safe abd feasible in patients with advanced non-small cell lung cancer who have had prior chemotherapy (abstract 7107). J Clin Oncol 23(16S):647s, 2005.
15. Gillenwater H, Tynan M, Natoli S, et al: Second-line gemcitabine in refractory stage IV non-small cell lung cancer: A phase II trial. Clin Lung Cancer 2:133-138, 2000.
16. Crino L, Mosconi A, Scagliotti G, et al: Gemcitabine as second-line treatment for advanced non-small cell lung cancer: A phase II trial. J Clin Oncol 17:2081-2085, 1999.
17. Socinski M, Schell M, Bakri K, et al: Second-line low-dose weekly paclitaxel in patients with stage IIIB/IV non-small cell lung cancer failing first-line carboplatin/paclitaxel. Cancer 95:1265-1273, 2002.
18. Lilenbaum R, Bonomi P, Ansari R, et al: A phase II Trial of cetuximab as therapy for recurrent non-small cell lung cancer (NSCLC): Final results (abstract 7036). J Clin Oncol 23(16S):629s, 2005.
19. Kim ES, Mauer AM, Tran HT, et al: A phase II study of cetuximab, an epidermal growth factor receptor (EGFR) blocking antibody in combination with docetaxel in chemotherapy refractory/resistant patients with advanced non-small cell lung cancer: Final report (abstract 2581). Proc Am Soc Clin Oncol 22:642, 2003.
20. Herbst RS, Johnson DH, Mininberg E, et al: Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol 23:2544-2555, 2005.
21. Sandler AB, Gray, R, Brahmer J, et al: Randomized phase II/III trial of paclitaxel (P) plus carboplatin (C) with or without bevacizumab in patients with advanced nonsqumous cell lung cancer (NSCLC): An Eastern Cooperative Oncology Group (ECOG) Trial–E4599 (abstract 4). J Clin Oncol 23(16S):2s, 2005.