Topoisomerase I Inhibitors in the Combined-Modality Therapy of Lung Cancer
Locally advanced non–small-cell lung cancer represents 30% to 40%of all pulmonary malignancies. Most patients will die of the diseaseafter aggressive contemporary treatments. Therefore, significant improvementin therapeutic methods must be implemented to improveoverall survival rates. The arrival of a new generation of chemotherapeuticagents-including the taxanes, gemcitabine (Gemzar), andtopoisomerase inhibitors such as irinotecan (Camptosar) and topotecan(Hycamtin)-offers the hope of significant advances in the treatmentof lung cancer. Irinotecan and topotecan are camptothecin derivativesthat inhibit topoisomerase I enzyme. It is believed that topoisomerase Iinhibitors stabilize a DNA/topoisomerase I complex and interact withreplication machinery to cause cell death. A significant amount of datademonstrates that these topoisomerase I inhibitors also act asradiosensitizers. With the increasing data that support concurrentchemoradiation treatment for malignancies, including lung cancer andhead and neck cancers, there is an impetus to pursue the additionaldrugs that may potentially improve local control and survival. Irinotecanis undergoing early clinical trials in the combined-modality setting inseveral different disease sites. This paper will review the data on therole of camptothecin derivatives as a radiosensitizer and as a componentof combined-modality therapy for lung cancer. It is hoped thatnewer treatment strategies, like the combination of radiation andtopoisomerase I inhibitors, will have a significant impact on cure ratesin the future.
ABSTRACT: Locally advanced nonâsmall-cell lung cancer represents 30% to 40%of all pulmonary malignancies. Most patients will die of the diseaseafter aggressive contemporary treatments. Therefore, significant improvementin therapeutic methods must be implemented to improveoverall survival rates. The arrival of a new generation of chemotherapeuticagents-including the taxanes, gemcitabine (Gemzar), andtopoisomerase inhibitors such as irinotecan (Camptosar) and topotecan(Hycamtin)-offers the hope of significant advances in the treatmentof lung cancer. Irinotecan and topotecan are camptothecin derivativesthat inhibit topoisomerase I enzyme. It is believed that topoisomerase Iinhibitors stabilize a DNA/topoisomerase I complex and interact withreplication machinery to cause cell death. A significant amount of datademonstrates that these topoisomerase I inhibitors also act asradiosensitizers. With the increasing data that support concurrentchemoradiation treatment for malignancies, including lung cancer andhead and neck cancers, there is an impetus to pursue the additionaldrugs that may potentially improve local control and survival. Irinotecanis undergoing early clinical trials in the combined-modality setting inseveral different disease sites. This paper will review the data on therole of camptothecin derivatives as a radiosensitizer and as a componentof combined-modality therapy for lung cancer. It is hoped thatnewer treatment strategies, like the combination of radiation andtopoisomerase I inhibitors, will have a significant impact on cure ratesin the future.In 2004, it is estimated that in theUnited States there will be 68,510deaths among women and 91,930deaths among men due to lung cancer,which is the leading cause of cancerdeaths in the United States. Lungcancer accounts for 14% of new cancercases and 28% of cancer deathsper year in the United States.[1] The5-year survival rate for lung cancershas been around 15% from 1974through 1995.[2] Most of these curesare related to surgical treatment ofpatients presenting with stage I and IIcancers. However, about 35% of patientspresent with locally advanceddisease that is not amenable to surgicaltherapy but may be potentiallycurable.[3] Traditional radiation treatmentalone in this group of patientshas yielded dismal cure rates at 5years. Therefore, a combined-modalitytherapy has been sought to improvethe poor outcome with singlemodality alone.Patients with clinical stage IIIAhave an overall 5-year survival rate of10% to 15%. However, this figuredrops to 2% to 5% when there is grosslyvisible disease in the mediastinumon a chest x-ray.[4] The principalforms of treatment for patients withstage III non-small-cell lung cancer(NSCLC) are radiation therapy, chemotherapy,surgery, and combinationsof these modalities.Several potential benefits are derivedfrom interactions of radiotherapyand chemotherapy to improve therapeuticoutcome. Combined radiotherapyand chemotherapy may increasetumor response, protect normal tissues,and exhibit nonoverlapping toxicities.[5] There is potential synergisticincrease in enhancement of tumori-cidal effect in specific anatomic siteswhere single-modality therapy mayhave limited efficacy. Chemotherapeuticagents may reduce the radiotherapy-induced normal tissue toxicityto an acceptable level for patients.Finally, two partially effective therapeuticmodalities may be combinedwithout having to significantly reducetheir dose levels to avoid treatmentrelatedtoxicities.
Multiple phase III trials have confirmedtherapeutic benefits of combiningchemotherapy and radiotherapyin locally advanced NSCLC, but withincreased treatment-related toxici-y.[6-9] A large meta-analysis of 22trials (3,033 patients and 2,814 deaths)comparing radiotherapy administeredalone or with chemotherapy demonstrateda 10% reduction in the relativerisk of death with chemoradiotherapyvs radiotherapy as a sole modality(P = .006), with an absolute reductionin deaths of 3% at 2 years and 2% at 5years.[10] Another meta-analysis fromPritchard et al suggested that traditionalchemotherapy added to radiotherapyadds an average of 2 monthsto patient survival.[11] Recent trialshave shown that concurrent chemoradiotherapyresults in a better overallsurvival compared with sequentialchemoradiotherapy in NSCLC.[12,13]Combined chemotherapy and radiotherapycurrently remains the standardtreatment for locally advancedNSCLC. However, local failure ratescan be around 80%.[6,9] Therefore,ongoing trials seek to improve theoutcome for treatment of lung cancer.Multiple agents including paclitaxel,docetaxel (Taxotere), vinorelbine,gemcitabine (Gemzar), and irinotecan(Camptosar) show a 20% to 54% responserate for single-agent treatmentin metastatic NSCLC.[3] This reviewwill focus on the data surrounding theuse of topoisomerase I inhibitors incombination with thoracic radiotherapyin the treatment of locally advancedNSCLC.CamptothecinCamptothecin is an alkaloid originallyfound in the Chinese tree Camptothecaacuminata. The US NationalCancer Institute first discovered camptothecinwith antitumor activity in the1960s.[14] In preclinical studies, theantitumor activity was seen againstcolon and gastric cancers and leukemia.However, unpredictable toxicities,including myelosuppression andhemorrhagic cystitis, were seen in patientstreated with camptothecin.Greater understanding of the mechanismsof action of camptothecin anddevelopment of water-soluble compoundsgenerated greater interest incamptothecin as a potential chemotherapeuticagent.Camptothecin and its derivativestarget topoisomerase I, the DNArelaxingenzyme.[15-18] Althoughtopoisomerase I was discovered in the1970s, its mechanism of action inDNA replication was not clearly understooduntil the 1980s. TopoisomeraseI was shown to be the targetof camptothecin in 1985 by Hsiang etal.[19] This enzyme serves to relaxboth positively and negatively supercoileddouble-helix DNA to allow replicationand transcription. It causesreversible single-strand breaks, whichallow rotation of the broken DNAstrand around the intact strand. Thecritical step for drug interaction is stabilizationof the topoisomerase I/DNAcomplex that the enzyme forms whencleaving DNA to allow for uncoilingto occur.[15-20] In the presence ofcamptothecin, a camptothecin/topoisomeraseI/DNA complex becomesstabilized because the 5'-phosphorylterminus of the enzyme-catalyzedDNA single-strand break is boundcovalently to a tyrosine residue of topoisomeraseI. These complexes arenonlethal and reversible.However, the single-strand breaksbecome irreversible double-strandbreaks when the DNA replication forkcollides with reversible complex duringS phase or during unscheduledDNA replication. The resulting celldeath can be recognized by the p53damage-sensing pathway and may resultin acceleration of apoptosis.[21-24] Thus, the cytotoxic effect of thecamptothecin requires active DNAreplication. In vitro studies haveshown that cells in S phase may be100 to 1,000 times more sensitive tocamptothecin than cells in the G1 orG2 phase of the cell cycle.[25]
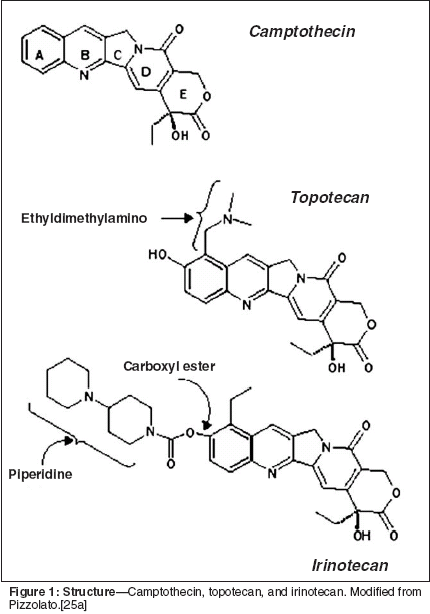
IrinotecanIn the 1970s, camptothecin provedto be too toxic as a chemotherapeuticagent. However, one of the water solublederivatives, irinotecan (7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin) or CPT-11(Figure 1) displayed antineoplasticactivity with improved toxicity profile.[26] Irinotecan was first commerciallyavailable in Japan in 1994 fortreatment of lung, cervical, and ovariancancers. Irinotecan is a prodrugthat is metabolized intracellullarly toits active metabolite, SN-38, by a carboxyesteraseconverting enzyme. Thismetabolite is over 1,000 times morepotent as an inhibitor of topoisomeraseI than irinotecan.[27,28] All of thecamptothecins have a terminal lactonering, which can be hydrolyzed toa less active form. However, underacidic conditions such as in the microenvironmentof a tumor, the activelactone species is favored.[27] Theplasma half-life of SN-38 after a shortintravenous infusion is approximately11.5 hours. Thus, a low concentrationof the metabolite may remain after2 days and have cytotoxic effect.[29]The major excretory pathway ofSN-38 is via hepatic glucuronidationand a decreased ability to glucuronidatemay possibly correlate withincreased gastrointestinal side effects.One of the dose-limiting toxicities ofirinotecan is delayed onset diarrheathat can be potentially life threatening.The diarrhea is felt to be relatedto the relatively high S-phase fractionof the intestinal mucosa, as well as tothe action of intestinal flora glucuronidasein cleaving the camptothecinglucuronidase conjugate leadingto the release of the drug in the intestinallumen.[30] Other commonly observedtoxicities include neutropenia,nausea, and vomiting.Interaction of CamptothecinDerivatives and RadiationSeveral investigators have reportedthat irinotecan enhances the cytotoxiceffect of radiation in vitro and invivo.[31,32] Omura et al[33] foundthat the cell kill was significantly enhancedwhen radiation was combinedwith the irinotecan derivative SN-38.The largest enhancement in cytotoxicitywas seen when irinotecan wasgiven just before or just after radiationtherapy. Their data suggested thatradiosensitization occurs by inhibitionof potentially lethal damage repair.[33] Chen et al[34] showed thathuman MCF-7 breast cancer cells thatwere exposed to 20(S)-10,11 methylenedioxycamptothecinbefore or duringradiation showed radiosensitizationratios of 1.6, while those treatedwith the drug after radiation showedsubstantially less enhancement of radiation-induced DNA damage.Kim et al also found greater radiosensitizationwhen topotecan (Hycamtin)was administered 2 to 4 hoursbefore radiation therapy compared to2 hours after radiotherapy in the treatmentof murine fibrosarcomas.[35]The radiobiology data imply that patientsshould be treated with a camptothecinderivative-based chemotherapyprior to or during their radiotherapyin order to derive full benefitsof combined-modality therapy. Dataalso suggest that camptothecin derivativesincluding 9-nitro-20 (S)-camptothecin,[36] 9-aminocamptothecin,[37] and topotecan[38] are ableto potentiate the tumoricidal effectsof radiation.There are several hypotheses aboutmechanism of interaction between radiationand irinotecan. The first hypothesissuggests that the inhibitionof topoisomerase I by camptothecinor its derivatives leads to the inhibitionof repair of radiation-inducedDNA strand breaks. The second hypothesissuggests that irinotecan or itsanalogs cause redistribution of cellsinto the more radiosensitive G2 phaseof the cell cycle. The third hypothesissuggests topoisomerase I/DNA adductsare trapped by irinotecan at thesites of radiation-induced singlestrandbreaks leading to their conversioninto double-strand breaks.[36]However, there is not sufficient evidenceto identify the underlying mechanismwith certainty. The predominanceof the particular mechanismthat is involved with radiosensitizationmay depend on which derivativeof camptothecin is being used in combined-modality therapy.Irinotecan in Combinationfor NSCLCBasic principles used in the selectionof chemotherapy drugs includenonoverlapping toxicities, differingmechanisms of action, and non-crossresistance.[39] Based on the abovecriteria, both preclinical and humandata address the combination of cisplatinand irinotecan in lung cancer.Kudoh et al showed that in xenograftsof the small-cell lung cancer (SCLC)tumor lines MNSUL and LX1, use ofirinotecan in combination with cisplatinleads to a larger reduction in tumorsize than either agent alone.[31]Early clinical studies in patientswith advanced NSCLC have yieldedfavorable response rates in excess of30%.[40] The combination of irinotecanand cisplatin has also been usedin phase I and II clinical trials; earlydata from phase II studies revealed a48% response rate in NSCLC[41] and78% for SCLC.[42] Ueoka et al[43]reported a phase I trial with fractionationof both the cisplatin and irinote-can. Cisplatin (60 mg/m2) was givenon days 1 and 8 and escalating dosesof irinotecan were given on the samedays. Each cycle was repeated every4 weeks. An impressive 78% responserate was seen in 18 patients withNSCLC.[43]A Vanderbilt-Ingram Cancer Centerphase II trial looking at the combinationof cisplatin at 80 mg/m2 on day1 and irinotecan at 60 mg/m2 on days1, 8, and 15 in 4-week courses withthe possibility of escalating the irinotecandose according to side effectswas also undertaken.[44] The finalirinotecan doses were modified to lessthan 40 mg/m2 with a response rate of29% in 52 patients. The median timeto progression was 4.4 months, with a1-year survival rate of 33%.Negoro et al[45] reported a randomizedphase III trial in which thecombination of irinotecan and cisplatinwas compared to irinotecan aloneor a cisplatin/vindesine combination.For stage IV patients, the irinotecan/cisplatin combination was superior tocisplatin/vindesine with respect to survival.Median survival times were 50.0and 36.4 weeks, respectively. No significantdifference in survival timewas seen between irinotecan/cisplatinand irinotecan alone. In addition, nosignificant difference in survival wasseen in stage IIIB disease. However,the authors noted that no particularrestriction was placed on chest irradiationin stage IIIB disease, whichmight have produced a heterogeneousgroup of IIIB patients.Masuda et al[46] reported a phaseI study of docetaxel and irinotecanfor stage IIIB and IV patients. Thirtytwopatients in the study were givenescalating doses of docetaxel andirinotecan starting with 30/40 mg/m2given at 4-week intervals in 10-mg/m2 increments until the maximum tolerateddose was reached. The maximumtolerated dose of docetaxel andirinotecan was 50/60 mg/m2 or 60/50mg/m2. Neutropenia and diarrhea werethe dose-limiting toxicities. There wasa partial response of 37% with a mediansurvival time of 48 weeks. Theauthors recommended 50 mg/m2 ofirinotecan on days 1, 8, and 15 and 50mg/m2 of docetaxel on day 2 givenevery 4 weeks for phase II trials.Irinotecan and Radiotherapyfor NSCLCCombined-modality treatment relieson the ability of radiation andchemotherapy to simultaneously addressboth local and micrometastaticdisease. An enhancement of local controlis due to the radiosensitizationeffects of concurrent chemotherapy.In addition, concurrent chemotherapyaddresses potential micrometastaticdisease that local therapy, such as radiotherapy,cannot address adequately.Understanding the basic mechanismsof interaction of different drugsis also important to maximize the tumoricidaleffects of chemotherapywhile minimizing treatment-relatedtoxicities.The optimal integration of irinotecanand cisplatin or other irinotecanbasedchemotherapy integrated withthoracic radiotherapy is unclear. Evidencefrom previously completed trialsaddresses sequencing of chemotherapyand radiotherapy in combinedmodality for lung cancer. In theCancer and Leukemia Group B(CALGB) 9130 trial, all patients receivedneoadjuvant platinum-basedchemotherapy followed by radiotherapywith randomization to concurrentcarboplatin (Paraplatin) with noimprovement in survival, but therewas a decreased local relapse rate.[47]The West Japan Lung CancerGroup has also compared concurrentand sequential combined-modalitytreatment in 314 patients with unresectablestage III NSCLC using a combinationof mitomycin (Mutamycin),vindesine, and cisplatin chemotherapy.Their results show a doubling of5-year survival rates (P = .03998) withconcurrent treatment.[48] Curran etal reported results of the RadiationTherapy Oncology Group's RTOG9410, which was a phase III, threearmtrial comparing standard sequentialchemoradiotherapy to twodifferent concurrent arms.[13] Thesequential arm used cisplatin at 100mg/m2 on days 1 and 29 with vinblastineat 5 mg/m2 weekly * 5 with 60Gy of thoracic radiotherapy followingthe chemotherapy. The second armused the same chemotherapy with 60Gy of thoracic radiotherapy startingon day 1. The third arm used cisplatinat 50 mg/m2 on days 1, 8, 29, and 36,with oral etoposide at 50 mg/m2 bidfor 10 doses on weeks 1, 2, 5, and 6,with thoracic radiotherapy of 69.6 Gyat 1.2 Gy bid starting on day 1.Acute toxicity was higher with theconcurrent treatment regimen, althoughlate toxicities were not differentbetween the arms. With medianfollow-up of 6 years, the arm withchemotherapy given concurrently withdaily radiotherapy showed a mediansurvival of 17 months (P = .046). Theabove trials support concurrent chemotherapyand radiotherapy for locallyadvanced NSCLC.Several phase I and II trials haveadministered irinotecan concomitantlywith thoracic radiotherapy in stageIII NSCLC (Table 1). Some trials addedother chemotherapeutic agents toirinotecan. The response rates in thesetrials are in excess of 60%. Thesecombinations appear to have reasonableacute toxicities; however, it istoo early to assess the late complicationrates.Takeda and colleagues examinedthe combination of escalating dosesof weekly irinotecan with concurrentthoracic radiotherapy (60 Gy in 30fractions over 6 weeks) in a phase I/IItrial for locally advanced NSCLC.[49]They enrolled patients with stage IIINSCLC who had an Eastern CooperativeOncology Group (ECOG) performancestatus of 0 to 2 and startedirinotecan at 30 mg/m2 intravenousweekly for 6 weeks. The maximumtolerated dose was 60 mg/m2. At thisdose level, five patients had grade 3/4toxicity, two had grade 3/4 esophagitis,and three had grade 3/4 pneumonitis.The irinotecan dose for thephase II portion of the trial was 45mg/m2. A further 10 patients weretreated at this dose level (17 total,including seven patients from phase Itrial). One of the 10 patients in thephase II portion developed pneumonitisand died, while another patient developedgrade 3 diarrhea. The overallresponse rate was 76.9%. After 22months of follow-up, 1-year survivalwas 61.5%.Saka and colleagues performed aphase II trial in which 24 patientswith locally advanced NSCLC wereenrolled and received irinotecan at 60mg/m2 intravenous weekly * 6 withconcurrent 60 Gy of thoracic radiationin 2-Gy/d fractions.[50] A totalof 71% of patients received theplanned chemotherapy, and 88% completedthe planned radiotherapy witha partial response rate of 79%. Toxicitiesincluded three (12.5%) cases ofgrade 3 pneumonitis, two (8.3%) casesof grade 3 esophagitis, two (8.3%)cases of grade 3 neutropenia, and one(4.2%) case of a grade 3 fever. Therewere no grade 4 toxicities.Choy et al reported results of aphase I trial of weekly irinotecan 30to 50 mg/m2 and concurrent radiotherapyfor unresectable stage IIINSCLC.[51] The response rate was58% among 13 treated patients. Nausea, vomiting, and esophagitis werethe major toxicities. The maximumtolerated dose of concurrent chest radiationtherapy and irinotecan was40 mg/m2 weekly for 6 weeks.
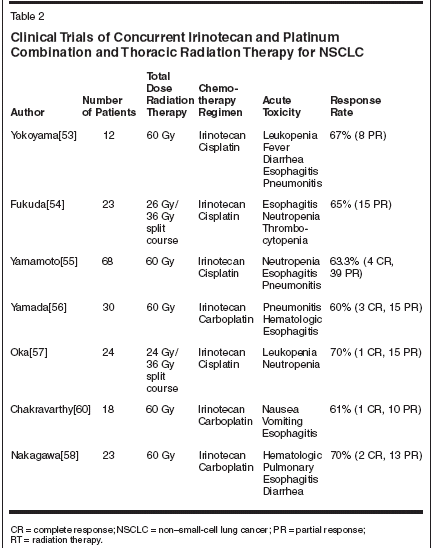
Kodoh and colleagues also performeda phase I/II trial of irinotecanand concurrent thoracic radiotherapyin locally advanced NSCLC.[52]The maximum tolerated dose was60 mg/m2 with dose-limiting toxicitiesof esophagitis, pneumonitis, anddiarrhea.Irinotecan/Platinum andThoracic Radiation TherapyPlatinum-based chemotherapyalong with radiotherapy has been wellestablished in the treatment of locallyadvanced NSCLC.[6-9] The evaluationof irinotecan and concurrent thoracicirradiation has expanded toinclude the incorporation of the platinumcompounds based on their knownactivity in NSCLC and preclinical data(Table 2).[31]
Yokoyama and colleagues in theJapan Clinical Oncology Group(JCOG) conducted a phase I trial of12 patients who received escalatingdoses of irinotecan and cisplatin with60 Gy of thoracic radiation.[53] Sixpatients were able to receive the level1 dose of 60 mg/m2 of cisplatin and40 mg/m2 of irinotecan with their radiation.However, chemotherapy wasdiscontinued before the planned threecycles were delivered in two patients.All patients completed the radiotherapy.At level 2 (60 mg/m2 of cisplatinand 60 mg/m2 of irinotecan), onlythree patients received all three cyclesof chemotherapy. The three patientswho did not completechemotherapy also did not completetheir radiotherapy. This group includedone patient who died after the secondcourse of chemotherapy. Due tothe low intensity of irinotecan in levels1 and 2 (irinotecan was often omittedon days 8 and 15 because ofneutropenia or diarrhea) and the lowradiation completion rate, the studywas closed at level 2. There was anoverall response rate of 67% (eight of12 patients had a partial response).However, the overall survival rate at1 year was only 33%.There have been two other reportedJapanese trials of concurrent cisplatin,irinotecan, and radiation inNSCLC. Fukuda et al were able togive two courses of chemotherapywith split-course radiation. Irinotecanat 60 mg/m2 on days 1, 8, and 15 andcisplatin at 80 mg/m2 on day 1 wasthe recommended dose for this phaseII study.[54] The overall response ratein 23 patients was 65%, with somecases of neutropenia, thrombocytopenia,and esophagitis.The Japanese Lung CancerGroup's (JLCG) follow-up study of68 patients involved induction cisplatinand irinotecan for two cycles followedby concurrent weekly irinotecanand thoracic radiation in patientswith unresectable stage IIINSCLC.[55] The significant toxicitieswere grade 4 neutropenia (6%),grade 3 esophagitis (4%), and grade 4pneumonitis (2%). The response ratewas 63.3%; the estimated 1-year survivalwas 71.7%.One other Japanese trial combined60 Gy thoracic radiation in 30 fractionswith carboplatin and irinotecan.[56] The 30 enrolled patientsreceived carboplatin at 20 mg/m2 dailyfor 5 days a week and intravenousirinotecan at 30 mg/m2 weekly. Bothdrugs were repeated for 4 weeks andthe dose of irinotecan was escalatedfrom 30 mg/m2 in 10-mg/m2 increments.The maximum tolerated doseof irinotecan was 60 mg/m2. The doselimitingtoxicities were pneumonitis,esophagitis, neutropenia, and thrombocytopenia.Among the 30 patients,there were three complete responsesand 15 partial responses, for an overallresponse rate of 60%. The mediansurvival was 14.9 months and the 2-year survival rate was 34.2%.Oka et al[57] reported results froma phase I trial of irinotecan and cisplatinwith concurrent split-course radiotherapyin patients with locallyadvanced stage III NSCLC. The overallresponse rate was 70%, with recommendeddoses for phase II studyof an irinotecan (60 mg/m2) and cisplatin(80 mg/m2) combination. Similarly,Nakagawa et al[58] reportedpreliminary results using an irinotecan/carboplatin combination in unresectablestage III NSCLC patients witha response rate of 70% among 23 patientsin the study.At the Vanderbilt-Ingram CancerCenter Affiliate Network, a phase IItrial for patients with stage III unresectableNSCLC was conducted. Themajor goals of this study were to determinethe maximum tolerated doseof irinotecan and carboplatin administeredwith radiation therapy, and toevaluate the toxicities of the combinationsof irinotecan and radiationtherapy and irinotecan/carboplatinand radiotherapy. Other secondaryobjectives were to evaluate the responserate and response duration ofadvanced, medically inoperable and/or surgically inoperable NSCLC treatedwith the combination of irinotecanand local irradiation or thecombination of carboplatin, irinotecan,and radiotherapy to the tumormasses.[59,60]The entry criteria for the trial requiredthe patients to have NSCLCwith unresectable stage III disease,including those with involved supraclavicularnodes with ECOG performancestatus 0 to 2 and less than 15%weight loss. In this trial, irinotecanwas administered as an intravenousinfusion, repeated every week for6 weeks with a starting dose of30 mg/m2. Doses were planned forescalation at 10-mg/m2 increments insuccessive cohorts of three patients.Thoracic radiotherapy was administeredto the primary tumor and regionallymph nodes (40 Gy) followedby a boost to the tumor (20 Gy).The results of the first 18 patientsentered onto this study through fourdose escalations of irinotecan (from30 to 50 mg/m2 weekly, including theaddition of carboplatin at an area underthe concentration-time curve[AUC] of 2, with 30 mg/m2 of irinotecan)were reported. One patient developedgrade 5 esophagitis at the firstdose level and the accrual was expandedto seven patients. No significantesophagitis was seen in the othersix patients. At the second dose levelof irinotecan (40 mg/m2/wk), one patientout of six experienced grade 2esophagitis. At the third dose level ofirinotecan (50 mg/m2/wk), two of threepatients entered developed grade 4nausea and vomiting, while two ofthe patients experienced grade 3 or 4esophagitis.There were 10 partial responsesand one complete response in 18 evaluablepatients for a response rate of61%. Nausea and vomiting as well asesophagitis were the main doselimitingtoxicities. These preliminarydata suggest that thoracic radiationcan be combined with weekly irinotecanand carboplatin with acceptabletoxicity, although results of higherdoses are not yet available. The responserates and survivals seen inthese phase I/II studies are encouragingand the toxicities associated withthe use of thoracic radiation and concurrentirinotecan are acceptable. Thistreatment strategy needs to be comparedwith other combined-modalityapproaches in locally advancedNSCLC in randomized phase II or IIItrials.These promising results with tolerabletoxicities in NSCLC shouldserve as a foundation to pursue theuse of combination irinotecan and radiationin other disease sites as well.Ongoing randomized trials in othersolid tumors will reveal additional informationregarding the effectivenessof the combination of irinotecan andradiotherapy.
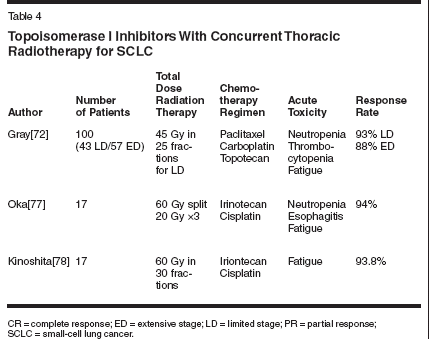
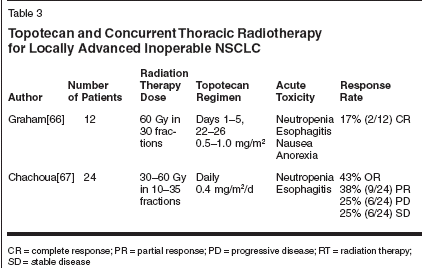
Topotecan in Combined-Modality Therapy for NSCLCIn patients with previously untreatedmetastatic NSCLC, single-agenttopotecan has yielded 0% to 15% rates,with the median survival duration of30 to 40 weeks.[61-63] Perez-Soler etal[61] initially found that topotecanhad an antitumor activity that appearedto be more effective for squamouscell carcinoma (36% response rate, 5of 14 patients) compared to adenocarcinoma(4% response rate, 1 of 26patients). However, a follow-up phaseII study[64] with 78 patients showedno significant difference between thehistologies when considering responseand survival end points.A randomized phase II trial of topotecanat 1.25 mg/m2/d * 5 every 3weeks and cisplatin at 75 mg/m2 onday 1 vs topotecan at 1.0 mg/m2/d * 5every 3 weeks and paclitaxel at 190mg/m2 on day 1 showed response ratesof 14% in the topotecan and cisplatinarm and 24% in the topotecan andpaclitaxel arm.[65] Unfortunately, theresults are not superior to other currentlyused combinations, and excessivetoxicity occurred in the topotecanand cisplatin arm.In 1993, Graham et al[66] conducteda phase I trial of topotecanwith concurrent radiotherapy for treatmentof patients with locally advancedinoperable NSCLC (Table 3). Topotecanwas administered by bolus infusionon days 1 to 5 and 22 to 26 atescalating doses: six patients received0.5 mg/m2, three patients received0.75 mg/md patients received1 mg/m2. Patients received thoracicirradiation to a total tumor doseof 60 Gy in 30 fractions. Twelve patientswere enrolled and evaluable.At the dose level of 0.5 mg/m2,none developed grade 4 hematologictoxicity; one of the six patients developedgrade 3 esophagitis. At the0.75 mg/m2 dose level, two of thethree patients developed grade 3/4nonhematologic toxicity, which includedanorexia, fatigue, nausea, vomiting,dysphagia, and weakness; nonedeveloped grade 4 hematologic toxicity.At the 1.0-mg/m2 dose level, allthree patients developed dose-limitingtoxicities, including grade 3 esophagitisin one patient and grade 4neutropenia in two patients. Thus,dose-limiting toxicity was exceededat the 0.75 mg/m2 level with concurrentthoracic radiotherapy.Median survival of all patients was8.6 months. With a follow-up of 12 to24 months, two patients were aliveand free of disease, three patients werealive with disease (two with distantmetastases and one with local diseaseand distant metastasis), and the remainingseven patients had died fromtheir disease. Therefore, the investigatorssuggested that 0.5 mg/m2 oftopotecan ' 5 days for two cycles,concurrent with thoracic radiotherapy,was a tolerable regimen. Whileonly phase I data, the overall survivalin this trial was disappointing.Chachoua et al[67] conducted aphase I trial combining an escalatingcontinuous intravenous infusion oftopotecan at 0.4 mg/m2/d with escalatingdoses of concurrent thoracicradiotherapy in the treatment of 24solid tumor patients (22 NSCLC, 1breast, 1 mesothelioma) (Table 3). Theradiotherapy dose and topotecan infusionduration were escalated in analternating fashion through the doselevels. At the 60 Gy radiation dose,only NSCLC patients were eligible.Fifteen patients were able to completethe planned radiotherapy andtopotecan infusion. Of the remainingnine patients, one patient was ineligible,four had hematologic toxicity, oneprogressed, one withdrew, and twodiscontinued therapy due to esophagitis.Nonhematologic toxicities includedgrade 3 esophagitis in twopatients, grade 3 nausea/vomiting inone patient, and grade 3 fatigue intwo patients. The overall response ratewas 43%, and the thoracic radiotherapyat 60 Gy with topotecan at 0.4 mg/m2/d for 42 days was recommendedas the appropriate dose for future studyby the authors.When considering the phase IIstudies of topotecan alone in advanceddisease in combination with the phaseI results of combined treatment in locallyadvanced disease, topotecanseems to have limited activity inNSCLC compared with other membersof the "new" generation of chemotherapies,which includesirinotecan.Role of Topoisomerase IInhibitors in SCLCSignificant activity of topotecanwith or without platinum-based agentsin extensive-stage disease (ED) SCLChas been demonstrated.[68-70] Twophase II trials have reported approximately37% response rates.[69,70]Therefore, topotecan makes an attractiveagent to investigate in the limited-stage disease (LD) SCLC. One ofthe advantages of topotecan in SCLCis that it has the unique ability to crossthe blood-brain barrier, making it activein the treatment of central nervoussystem (CNS) metastasis fromSCLC. For example, Manegold etal[71] demonstrated, in a phase IIstudy of topotecan in patients withSCLC and brain metastases failingfirst-line therapy, that of 16 evaluablepatients, some of whom had previouslyreceived brain irradiation, four(25%) complete responses and six(38%) partial responses were observed.The objective response rate ofCNS metastases was 63%. This findingis important for SCLC treatment,where CNS relapses can be high.The promising response rates inED lead to investigations in LD SCLCpatients (Table 4). Gray et al[72] conducteda phase II trial evaluating athree-drug regimen (paclitaxel/carboplatin/topotecan) in the first-line treatmentof limited-stage SCLC. Patientsreceived four courses, and responderscontinued therapy with a furtherthree courses of oral etoposide. Patientswith LD SCLC received 45 Gy(1.8 Gy/d) to the chest beginning onweek 6 of chemotherapy. A total of100 patients were treated, including43 LD and 57 ED patients. Eightysevenpatients completed four coursesof the paclitaxel/carboplatin/topotecan combination. The responserates were 88% for ED patients and93% for LD patients, for an overallresponse rate of 90%. However, themedian survival for patients with LDwas not reported.Single-agent irinotecan and thecombination of irinotecan/cisplatin areboth active in the treatment ofSCLC.[73-75] A phase III trial fromthe Japan Clinical Oncology Groupshowed that the combination of cisplatinand irinotecan had survival advantageover the traditional standardtherapy of cisplatin and etoposide inED chemotherapy-naive patients.[76]The irinotecan/cisplatin regimen hada median survival of 12.8 months, vs9.4 months (P = .002) for the etoposideand cisplatin combination. Thetrial was stopped early because of thesignificant survival difference betweenthe two groups.Because both irinotecan and cisplatinare known radiosensitizers,these agents may also be beneficialwhen combined with concurrent radiotherapyfor the treatment of LD SCLC.Oka et al reported a phase I study ofirinotecan and cisplatin with concurrentsplit-course radiotherapy in LDSCLC.[77] Chemotherapy consistedof four cycles of irinotecan on days 1,8, and 15 and cisplatin on day 1, with20 Gy radiotherapy at 2 Gy/d givenon day 2 of each chemotherapy cyclefor a total cumulative dose of 60 Gyto the chest. Three dose levels of irinotecanwere 40, 50, and 60 mg/m2 witha constant cisplatin dose of 60 mg/m2.Of the 17 patients enrolled, 16 wereevaluable. There were four patientswith complete response, 11 patientswith partial response, and 1 patientwithout change. The overall responserate was 94%. Irinotecan at 40 mg/m2with cisplatin at 60 mg/m2 was recommendedfor phase II study.Kinoshita et al[78] conducted aphase I trial of escalating doses ofirinotecan and a constant dose of cisplatinwith concurrent thoracic radiotherapy(60 Gy) in patients with LDSCLC. Of the 17 patients enrolled, 16were evaluable. Nearly all patients responded,with 4 complete responsesand 11 partial responses, for an overallresponse rate of 93.8%. The investigatorsrecommended that irinotecanbe administered at 40 mg/m2 in futurestudies, as two of four patients whoreceived irinotecan 60 mg/m2 discontinuedtherapy due to severe fatigue.The dose intensity of irinotecan at 50mg/m2 was only 49%. This data cer-tainly suggests that this combinationdeserves further pursuit in a randomizedphase II trial.Newer Topoisomerase IInhibitorsPhase II clinical trials of 9-aminocamptothecinand exatecan mesylate(DX-8951f) have tested the efficacyof these new topoisomerase I inhibitorsin advanced NSCLC patients. 9-aminocamptothecin showed modestsingle-agent activity in NSCLC withan 8.6% response rate (5 partial responsesout of 58 patients) and a mediansurvival for the entire study groupof 5.4 months.[79] Certainly its demonstratedradiosensitizing abilities[80]make 9-aminocamptothecin an attractivecandidate for further study in earlierstages of disease.In another phase II trial, exatecanmesylate yielded an 18% overall responserate (3 partial responses out of16 patients).[81] Using these phase IItrials as a guide, it may be reasonableto pursue further study of these newtopoisomerase I inhibitors in locallyadvanced NSCLC.ConclusionThere has been improvement inoutcomes of locally advanced NSCLCusing combined-modality therapy.Concurrent chemoradiation approachhas accumulated significant data tosupport its application in lung cancer.The response rates and survival rateswith irinotecan and radiation inNSCLC from phase I/II studies are encouraging,and the toxicities associatedwith the use of thoracic radiation andconcurrent irinotecan are manageable.This treatment strategy needs to be comparedwith other combined-modalityapproaches in locally advanced NSCLCin randomized trials.These results of combined radiationand topoisomerase I inhibitors inthe treatment of lung cancer shouldalso encourage the study of combinationirinotecan and topotecan with radiationin other disease sites as well.The activity of irinotecan in colorectalcancers suggests that this could bean area in which to exploit the potentialof irinotecan as a radiosensitizer.Ongoing trials are testing camptothecinderivatives with concurrent radiationin other solid tumors. The resultsof ongoing trials will provide a greaterunderstanding in pursuit of optimalcombined-modality therapy.
Disclosures:
Dr. Choy has receivedresearch support from Aventis. He has servedon speakers’ bureaus and acted as a consultantfor Amgen, Bristol-Myers Squibb,AstraZeneca, Aventis, Pfizer, and Lilly. He hasserved on speakers’ bureaus for OSI,Genentech, and MedImmune.
References:
1.
American Cancer Society: Cancer Factsand Figures 2004. Atlanta, American CancerSociety, 2004.
2.
Greenlee RT, Murray T, Bolden S, et al:Cancer Statistics, 2000. CA Cancer J Clin50(1):7-33, 2000.
3.
Stevens CW, Lee JS, Cox J, et al: Novelapproaches to locally advanced unresectablenon-small cell lung cancer. Radiother Oncol55(1):11-8, 2000.
4.
Komaki R, Cox JD, Hartz AJ, et al: Characteristicsof long-term survivors after treatmentfor inoperable carcinoma of the lung. AmJ Clin Oncol 8(5):362-370, 1985.
5.
Steele GG, Peckham MJ: Exploitablemechanisms in combined radiotherapy-chemotherapy:The concept of additivity. Int J RadiatOncol Biol Phys 5:85-91, 1979.
6.
Dillman RO, Herndon J, Seagren SL, etal: Improved survival in stage III non-smallcelllung cancer: Seven year follow up of Cancerand Leukemia Group B 8433 trial. J NatlCancer Inst 88:1210-1215, 1996.
7.
Schaakae-Koning C, Vanden Bogart W,Dalesio O: Effects of concomitant cisplatin andradiotherapy on inoperable non-small cell lungcancer. N Engl J Med 326:524-530, 1992.
8.
Sause WT, Scott C, Taylor S, et al: RadiationTherapy Oncology Group (RTOG) 88-08and Eastern Cooperative Oncology Group(ECOG) 4588: Preliminary results of a phaseIII trial in regionally advanced, unresectablenon-small cell lung cancer. J Natl Cancer Inst87:198-205, 1995.
9.
LeChevalier T, Arriagada R, Quoix E, etal: Radiotherapy alone versus combined chemotherapyand radiotherapy in nonresectablenon-small-cell lung cancer: First analysis of arandomized trial in 353 patients. J Natl CancerInst 83:417-423, 1991.
10.
Non-small Cell Lung Cancer CollaborativeGroup: Chemotherapy in non-small celllung cancer: A meta-analysis using updateddata on individual patients from 52 randomizedclinical trials. Br Med J 311:899-909,1995.
11.
Pritchard RS, Anthony SP: Chemotherapyplus radiotherapy compared with radiotherapyalone in the treatment of locallyadvanced, unresectable, non-small cell lungcancer. A meta-analysis. Ann Intern Med125:723-729, 1996.
12.
Furuse K, Fukuoka M, Kawahara, M, etal: Phase III study of concurrent versus sequentialthoracic radiotherapy in combination withmitomycin, vindesine, and cisplatin inunresectable stage III non-small-cell lung cancer.J Clin Oncol 17:2692-2699, 1999.
13.
Curran WJ, Scott CB, Langer JL,et al:Long-term benefit is observed in a phase IIIcomparison of sequential vs. concurrentchemo-radiation for patients with unresectablestage III NSCLC. RTOG 9410 (abstract 2499).Proc Am Soc Clin Oncol 22:21, 2003.
14.
Wall ME, Wani MC, Cook CE, et al:Plant antitumor agents, I: The isolation andstructure of camptothecin, a novel alkaloidalleukemia and tumor inhibitor fromCamptotheca acuminata. J Am Chem Soc88:3888-3890, 1966.
15.
Hsiang Y-H, Hertzberg R, Hecht S, et al:Camptothecin induces protein-linked DNAbreaks via mammalian DNA topoisomerase I.J Biol Chem 260:14873-14878, 1985.
16.
Hsiang Y-H, Liu LF: Identification ofmammalian DNA topoisomerase I as an intracellulartarget of the anticancer drugcamptothecin. Cancer Res 48:1722-1726,1988.
17.
Andoh T, Ishii K, Suzuki Y, et al: Characterizationof a mammalian mutant with acamptothecin resistant DNA topoisomerase I.Proc Natl Acad Sci USA 84:5565-5569, 1987.
18.
Nitiss J, Wang JC: DNA topoisomerasetargetingantitumor drugs can be studied inyeast. Proc Natl Acad Sci USA 85:7501-7505,1988.
19.
Hsiang YH, Hertzberg R, Hecht S, et al:Campothecin induces protein-linked DNAbreaks via mammalian DNA topoisomerase I.J Biol Chem 260:1483-1478, 1986.
20.
Chen AY, Yu C, Gatto B, et al: DNA minorgroove-binding ligands: A different classof mammalian DNA topoisomerase I inhibitors.Proc Natl Acad Sci USA 90:8131-8135, 1993.
21.
Li LH, Fraser TJ, Olin EJ, et al: Actionof camptothecin on mammalian cells in culture.Cancer Res 32:2643-2650, 1972.
22.
Lamond J, Wang M, Kinsella T, et al:Radiation lethality enhancement with 9-aminocamptothecin: Comparison to othertopoisomerase I inhibitors. Int J Radiat OncBiol Phys 36(2):369-376, 1996.
23.
Nelson WG, Kastan MB: DNA strandbreaks: The DNA template alterations that triggerp53-dependent DNA damage responsepathways. Mol Cell Biol 14:1815-1823, 1994.
24.
Tishler RB, Calderwood SK, ColemanCN, et al: Increases in sequence specific DNAbinding by p53 following treatment with chemotherapeuticand DNA damaging agents.Cancer Res 53:2212-2216; 1993.
25.
Li LH, Fraser TJ, Olin EJ, et al: Actionof camptothecin on mammalian cells in culture.Cancer Res 32:2643-2650, 1972.
25a.
Pizzolato JF, Saltz LB: Thecamptothecins. Lancet 361:2235-2242, 2003.
26.
Chen AY, Leroy FL: DNATopoisomerases: Essential enzymes and lethaltargets. Annu Rev Pharmacol Toxicol 34:191-218, 1994.
27.
Takimoto CH, Wright J, Arbuck SG:Clinical applications of the camptothecins.Biochim Biophys Acta 1400(1-3):107-119,1998.
28.
Kawato Y, Aonuma M, Hirota Y, et al:Intracellular roles of SN-38, a metabolite of thecamptothecin derivative CPT-11, in the antitumoreffect of CPT-11. Cancer Res 51:4187-4191, 1991.
29.
Rothenberg ML, Kuhn JG, Burris HAIII, et al: Phase I and pharmocokinetic trial ofweekly CPT-11. J Clin Oncol 11:2194-2204,1993.
30.
Araki E, Ishikawa M, Iigo M, et al: Relationshipbetween development of diarrheaand the concentration of SN-38, an active metaboliteof CPT-11, in the intestine and theblood plasma of athymic mice following intraperitonealadministration of CPT-11. Jpn JCancer Res 84(6):697-702, 1993.
31.
Kudoh S, Takada M, Masuda N, et al:Enhanced antitumor efficacy of a combinationof CPT-11, a new derivative of camptothecin,and cisplatin against human lung tumor xenografts.Jpn J Cancer Res 84(2):203-207,1993.
32.
Rich TA, Kirichenko AV: Camptothecinradiation sensitization: Mechanisms, schedules,and timing. Oncology (Huntingt) 12(8 suppl6):114-120, 1998.
33.
Omura M, Torigoe S, Kubota N: SN-38,a metabolite of the camptothecin derivativeCPT-11, potentiates the cytotoxic effect of radiationin human colon adenocarcinoma cellsgrown as spheroids. Rad Oncol 43:197-201,1997.
34.
Chen AY, Okunieff P, Pommier Y, et al:Mammalian DNA topoisomerase I mediates theenhancement of radiation cytotoxicity bycamptothecin derivatives. Cancer Res57(8):1529-1536, 1997.
35.
Kim JH, Kim SH, Kolozsvary A, et al:Potentiation of radiation response in humancarcinoma cells in vitro and murine fibrosarcomain vivo by topotecan, an inhibitor of DNAtopoisomerase I. Int J Radiat Oncol Biol Phys22:515-518, 1992.
36.
Amorino GP, Hercules SK, Mohr PJ, etal: Preclinical evaluation of the orally activecamptothecin analog, RFS-2000 (9-nitro-20(S)-camptothecin) as a radiation enhancer.Int J Radiat Oncol Biol Phys 47(2):503-509,2000.
37.
Lamond JP, Wang M, Kinsella TJ, et al:Radiation lethality enhancement with 9-aminocamptothecin: Comparison to othertopoisomerase I inhibitors. Int J Radiat OncolBiol Phys 36(2):369-376, 1996.
38.
Mattern MR, Hofmann GA, McCabe Fl,et al: Synergistic cell killing by ionizing radiationand topoisomerase I inhibitor topotecan(SK&F 104864). Cancer Res 51(21):5813-5816, 1991.
39.
De Vita VT, Hellman S, Rosenberg SA(eds): Cancer: Principles and Practice of Oncology,6th ed, pp 289-307. Philadelphia,Lippincott-Raven, 2000.
40.
Fukuoka M, Niitani H, Suzuki A, et al:A Phase II study of CPT-11, a new derivativeof camptothecin for previously untreated nonsmall-cell lung cancer. J Clin Oncol 10(1):16-20, 1992.
41.
Nakagawa K, Fukuoka M, Nitiani H, etal: Phase II study of irinotecan (CPT-11) andcisplatin in patients with advanced non-smallcelllung cancer(NSCLC) (abstract). Proc AmSoc Clin Oncol 12:332, 1993.
42.
Fujiwara Y, Yamakido M, Fukuoka M,et al: Phase II study of irinotecan (CPT-11) andcisplatin (CDDP) in patients with small celllung cancer (SCLC) (abstract). Proc Am SocClin Oncol 13:335, 1994.
43.
Ueoka H, Tabata M, Kiura K, et al: Fractionatedadministration of irinotecan andcisplatin for treatment of lung cancer: A phaseI study. Br J Cancer 79(5-6):984-990, 1999.
44.
Devore RF, Johnson DH, Crawfors J, etal: Phase II study of irinotecan plus cisplatinin patients with advanced non-small-cell lungcancer. J Clin Oncol 17(9):2710-2720, 1999.
45.
Negoro S, Masuda N, Takada Y, et al:Randomized phase III trial of irinotecan combinedwith cisplatin for advanced non-smallcelllung cancer. Br J Cancer 88:335-341, 2003.
46.
Masuda N, Negoro S, Kudoh S, et al:Phase I and pharmacologic study of docetaxeland irinotecan in advanced non-small-cell lungcancer. J Clin Oncol 18(16):2996-3003, 2000.
47.
Clamon G, Herndon J, Cooper R, et al:Radiosensitization with carboplatin for patientswith unresectable stage III non-small-cell lungcancer: A phase III trial of the Cancer and LeukemiaGroup B and the Eastern CooperativeOncology Group. J Clin Oncol 17(1):4-11,1999.
48.
Furuse K, Fukuoka M, Kawahara M, etal: Phase III study of concurrent versus sequentialthoracic radiotherapy in combination withmitomycin, vindesine, and cisplatin inunresectable stage III non-small-cell lung cancer.J Clin Oncol 17(9):2692-2699, 1999.
49.
Takeda K, Negoro S, Kudoh S, et al:Phase I/II study of weekly irinotecan and concurrentradiation therapy for locally advancednon-small cell lung cancer. Br J Cancer 79(9-10):1462-1467, 1999.
50.
Saka H, Shimokata K, Yoshida S, et al:Irinotecan (CPT-11) and concurrent radiotherapyin locally advanced non-small cell lungcancer (NSCLC): A phase II study of JapanClinical Oncology Group (JCOG9504) (abstract1607). Proc Am Soc Clin Oncol 16:447a,1997.
51.
Choy H, Chakravarthy A, Devore RF, etal: Weekly irinotecan and concurrent radiationtherapy for stage III unresectable NSCLC.Oncology 14:43-46, 2000.
52.
Kodoh S, Kurihara N, Okishio K, et al:A phase I/II study of weekly irinotecan (CPT-11) and simultaneous thoracic radiotherapy(TRT) for unresectable locally advanced nonsmallcell lung cancer (NSCLC) (abstract). ProcAm Soc Clin Oncol 15:1102, 1996.
53.
Yokoyama A, Kurita Y, Saijo N, et al:Dose-finding study of irinotecan and cisplatinplus concurrent radiotherapy for unresectablestage III non-small-cell lung cancer. Br J Cancer78(2):257-262, 1998.
54.
Fukuda M, Fukuda Mi, Soda H, et al:Phase I study of irinotecan (CPT-11) andcisplatin (CDDP) with concurrent thoracic radiotherapyin locally advanced non-small celllung cancer (NSCLC) (abstract 1796). Proc AmSoc Clin Oncol 18:466a, 1999.
55.
Yamamoto N, Fukuoka M, Negoro S, etal: A phase II study of induction chemotherapywith CPT-11 and cisplatin followed by thoracicradiation combined with weekly CPT-11 inpatients with unresectable stage III non-smallcell lung cancer (abstract 1953). Proc Am SocClin Oncol 19:499a, 2000.
56.
Yamada M, Kudoh S, Fukuda H, et al:Dose escalation study of weekly irenotecan anddaily carboplatin with concurrent thoracic radiotherapyfor unresectable stage III non-smallcell lung cancer. Br J Cancer 87(3):258-263,2002.
57.
Oka M, Fukuda M, Fukuda M, et al:Phase I study of irinotecan and cisplatin withconcurrent split-course radiotherapy inunresectable and locally advanced non-smallcell lung cancer. Eur J Cancer 37:1359-1465,2001.
58.
Nakagawa K, Yamamoto S, Kudoh S, etal: Irinotecan (CPT-11) and carboplatin(CBDCA) with concurrent thoracic radiotherapy(TRT) for unresectable, stage III nonsmall-cell lung cancer (NSCLC): Preliminaryresults (abstract). Proc Am Soc Clin Oncol17:496a, 1998.
59.
Chakravarthy A, Choy H: A phase I trialof outpatient weekly irinotecan/carboplatin andconcurrent radiation for stage III unresectablenon-small-cell lung cancer: A Vanderbilt-Ingram Cancer Center Affiliate Network trial.Clin Lung Cancer 1(4):310-311, 2000.
60.
Chakravarthy A, Choy H, De Vore RD,et al: Phase I trial of outpatient weeklyirinotecan and carboplatin with concurrent radiationtherapy for stage III unresectable nonsmallcell lung cancer: A Vanderbilt CancerCenter Affiliate Network Trial (VCCAN) trial(abstract 2040). Proc Am Soc Clin Oncol19:511a, 2000.
61.
Perez-Soler R, Fossella FV, Glisson BS,et al: Phase II study of topotecan in patientswith advanced non-small-cell lung cancer previouslyuntreated with chemotherapy. J ClinOncol 14:503-513, 1996.
62.
Lynch TJ, Kalish L, Strauss G, et al:Phase II study of topotecan in metastatic nonsmall-cell lung cancer. J Clin Oncol 12:347-352, 1994.
63.
Kindler HL, Kris MG, Smith IE, et al:Phase II trial of topotecan administered as a21-day continuous infusion in previously untreatedpatients with stage IIIB and IV nonsmall-cell lung cancer. Am J Clin Oncol 21:438-441, 1998.
64.
Weitz JJ, Marschke RF, Sloan JA, et al:A randomized phase II trial of two schedulesof topotecan for the treatment of advanced stagenon-small cell lung cancer. Lung Cancer28:157-162, 2000.
65.
Wiesenfeld M, Marks R, Grill J, et al: Arandomized phase II study of topotecan (TOPA)and cisplatin (CDDP) with filgrastim (G-CSF)versus topotecan and paclitaxel (Taxol) withfilgrastim in patients with advanced non-smallcell lung cancer (abstract 1755). Proc Am SocClin Oncol 16:488a, 1997.
66.
Graham MV, Jahanzeb M, Dresler CM,et al: Results of a trial with topotecan dose escalationand concurrent thoracic radiationtherapy for locally advanced, inoperable nonsmallcell lung cancer. Int J Radiat Oncol BiolPhys 36:1215-1220, 1996.
67.
Chachoua A, Hochster H, Steinfeld A,et al: Feasibility of seven weeks concomitanttopotecan (T) continuous infusion with thoracicradiation (abstract 1873). Proc Am Soc ClinOncol 18:485a, 1999.
68.
Ardizzoni A, Hansen H, DombernowskyP, et al: Topotecan, a new active drug in thesecond-line treatment of small-cell lung cancer:A phase II study in patients with refractoryand sensitive disease. The European Organization for Research and Treatment of CancerEarly Clinical Studies Group and New DrugDevelopment Office, and the Lung CancerCooperative Group. J Clin Oncol 15:2090-2096, 1997.
69.
Schiller JH, Kim K, Hutson P, et al: PhaseII study of topotecan in patients with extensive-stage small-cell carcinoma of the lung: AnEastern Cooperative Oncology Group trial. JClin Oncol 14:2345-2352, 1996.
70.
von Pawel J, Schiller JH, Shepherd FA,et al: Topotecan versus cyclophosphamide,doxorubicin, and vincristine for the treatmentof recurrent small-cell lung cancer. J Clin Oncol17:658-667, 1999.
71.
Manegold C, von Pawel J, ScheithauerW, et al: Response of SCLC brain metastaseson topotecan (SK&F 104864) therapy. AnnOncol 7(suppl 5):106-107, 1996.
72.
Gray JR, Hainsworth JD, Burris HA, etal: Paclitaxel, carboplatin, and topotecan in thetreatment of small cell lung cancer: A phase IItrial of the Minnie Pearl Cancer Research Network(abstract 1931). Proc Am Soc Clin Oncol19:494a, 2000.
73.
Masuda N, Fukuoka M, Kusunoki Y, etal: CPT-11: A new derivative of camptothecinfor the treatment of refractory or relapsed smallcelllung cancer. J Clin Oncol 10:1225-1229,1992.
74.
DeVore RF, Blanke CD, Denham CA, etal: Phase II study of irinotecan (CPT-11) inpatients with previously treated small-cell lungcancer (SCLC) (abstract 1736). Proc Am SocClin Oncol 17:451a, 1998.
75.
Kudoh S, Fujiwara Y, Takada Y, et al:Phase II study of irinotecan combined withcisplatin in patients with previously untreatedsmall-cell lung cancer. West Japan Lung CancerGroup. J Clin Oncol 16:1068-1074, 1998.
76.
Noda K, Nishiwaki Y, Kawahara M, etal: Irinotecan plus cisplatin compared withetoposide plus cisplatin for extensive small celllung cancer. N Engl J Med 346:85-91, 2002.
77.
Oka M, Fukuda M, Kuba M, et al: PhaseI study of irinotecan and cisplatin with concurrentsplit-course radiotherapy in limited-diseasesmall-cell lung cancer. Eur J Cancer38:1998-2004, 2002.
78.
Kinoshita A, Fukuda M, Kuba M, et al:Phase I study of irinotecan (CPT-11) andcisplatin (CDDP) with concurrent thoracic radiotherapy(TRT) in limited-stage small celllung cancer (LS-SCLC) (abstract 1999). ProcAm Soc Clin Oncol 19:511a, 2000.
79.
Vokes EE, Ansari RH, Masters GA, etal: A phase II study of 9-aminocamptothecinin advanced non-small-cell lung cancer. AnnOncol 9:1085-1090, 1998.
80.
Amorino GP, Hercules SK, Mohr PJ, etal: Preclinical evaluation of the orally activecamptothecin analog, RFS-2000 (9-nitro-20(S)-camptothecin) as a radiation enhancer.Int J Radiat Oncol Biol Phys 47:503-509, 2000.
81.
Talbot DC, White S, Jones P, et al: PhaseII study of exatecan mesylate (DX-8951f) inadvanced NSCLC (abstract 2166). Proc Am SocClin Oncol 19:549a, 2000.