Updates on the Management of Breast Cancer Brain Metastases
In this overview, we will review recent developments in the management of breast cancer brain metastases and current prospective trials of systemic therapies specifically for patients with breast cancer brain metastases, with a focus on novel pathway-specific therapies.
Table 1: Chemotherapy Agents Undergoing Evaluation for the Treatment of BCBMs
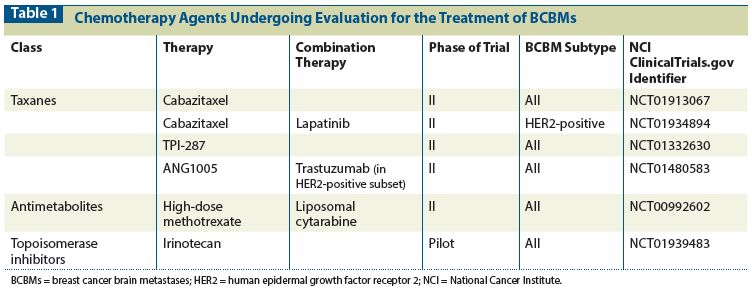
Table 2: Novel Therapeutic Strategies Undergoing Evaluation for the Treatment of BCBMs
Breast cancer brain metastases (BCBMs) are common in patients with advanced disease. Breast cancer subtype and performance status are the major determinants of the course of the disease and survival time following a diagnosis of brain metastasis. Unique challenges specific to the management of BCBMs include overcoming the blood-brain barrier and resistance to conventional systemic therapies, as BCBMs typically occur in the pretreated patient population. The development of new systemic therapies for breast cancer, coupled with improvements in trial design, imaging modalities, and methods of defining and measuring clinical endpoints, has led to a renewed interest in developing novel therapeutic approaches for BCBMs. In this overview, we will review recent developments in the management of BCBMs and current prospective trials of systemic therapies specifically for patients with BCBMs, with a focus on novel pathway-specific therapies.
Introduction
A diagnosis of central nervous system (CNS) recurrence is not uncommon in patients with breast cancer; an estimated 10% to 30% of all breast cancer patients will eventually develop brain metastases.[1] The diagnosis of breast cancer brain metastases (BCBMs) is associated with the shortest survival time compared with other sites of metastatic spread.[2]
The primary determinants of outcomes in patients with BCBMs are the tumor subtype and performance status of the patient.[3,4] In patients with early-stage breast cancer, the cumulative incidence rates of brain metastasis are highest in those with human epidermal growth factor receptor 2 (HER2)-positive and triple-negative breast cancer (TNBC; defined as estrogen receptor [ER]-negative, progesterone receptor [PR]-negative, and HER2-negative disease) and lowest in ER-positive disease.[2] A recent large retrospective study of 865 patients with BCBMs reported the median time interval from primary diagnosis to development of BCBMs, as well as median survival following the diagnosis of BCBMs, to be shortest in TNBC (27.5 months and 7.3 months, respectively) and HER2-positive disease (35.8 months and 17.9 months, respectively), and relatively longer in patients with ER-positive/HER2-negative (54.4 months and 10 months, respectively) and ER-positive/HER2-positive disease (47.4 months and 22.9 months, respectively).[5] Therefore, there is a great deal of interest in developing new therapeutic strategies for BCBMs, particularly in the TNBC and HER2-positive breast cancer subtypes.
A unique hurdle in the development of therapies for BCBMs is the presence of the blood-brain barrier (BBB), a tight layer of endothelial cells and astrocyte foot processes that acts as a selective barrier to the diffusion of systemic therapies.[6] The BBB is also characterized by the presence of drug efflux mechanisms, such as P-glycoprotein, a multidrug transporter.[7] Good penetration across the BBB is not always necessary for CNS activity, as the therapeutic effect is also dependent on other properties of the drug and the inherent sensitivity of the tumor.[8] Intrathecal drug administration may represent a direct route into the CNS, although such an approach would have to be combined with systemic administration, especially in the common setting of concurrent extracranial disease. In addition, in the case of deep intraparenchymal metastases, it is not clear whether intrathecal approaches would lead to adequate drug penetration.
Another challenge facing patients with BCBMs is that CNS recurrence typically occurs in the setting of failure of systemic treatment. Likely explanations for this include de novo resistance; acquired resistance to prior systemic treatment and radiotherapy; and an inability to penetrate the BBB, resulting in low CNS drug levels. While the intrinsic sensitivity of tumor cells to the pharmacologic agent is likely the most important determinant of therapeutic success, potential areas for development of treatment for BCBMs may include improving BBB penetration by disrupting the blood-tumor barrier or developing therapies capable of permeating the CNS, and evaluating novel therapies earlier in the treatment trajectory, rather than in a heavily pretreated setting.
The routine use of effective HER2-directed therapies has altered the natural history of HER2-positive breast cancer. The CNS as a site of first relapse is uncommon in patients who have received adjuvant HER2-directed systemic therapies (approximately 2% with trastuzumab in the HERA trial and approximately 1% with lapatinib in the TEACH trial).[9,10] However, 30% to 55% of patients with metastatic HER2-positive disease will eventually develop CNS metastases.[2,11-13] Interestingly, CNS recurrences tend to be widely distributed over time. Thus, we believe that early therapeutic interventions applied over a short time window in the metastatic setting are unlikely to prevent brain metastases from appearing later in a patient’s disease course. An exception to this may be adjuvant therapy; in this setting, however, the event rate is sufficiently low that testing a preventive agent is not practical unless we are able to identify strong predictors of early CNS recurrence.
Like patients with HER2-positive disease, patients with TNBC also have a high risk of CNS relapse (25% to 46%).[2,14,15] However, BCBMs in patients with TNBC differ from those associated with the HER2-positive subtype in that concurrent extracranial disease progression is common, and it is more likely to occur in the early phase of the disease course.[14,16,17] As a result, TNBC patients with BCBMs rarely die from progressive CNS disease alone-unlike HER2-positive patients with BCBMs, up to 50% of whom die from progressive CNS disease.[14] There is therefore an urgent need to develop additional systemic therapies that are effective in controlling intra-CNS and extra-CNS disease concurrently.
On a positive note, metastasis of breast cancer to the brain is no longer a clinical diagnosis for which therapeutic options and clinical trials are lacking. Improvements in systemic therapies and CNS-directed local therapies have likely improved patient outcomes, even after the development of CNS recurrence, and particularly in the HER2-positive subtype. With the establishment of standardized approaches to the assessment of post-treatment CNS response and outcomes, patients with BCBMs who were once routinely excluded from clinical trials now have available to them an increasing array of trials investigating novel approaches specific to BCBMs.
Current Treatment Strategies for BCBMs
Key determinants in the management of symptomatic BCBMs include the number, size, and site of lesions; the status of extracranial metastases; and the performance status of the patient. Most current management algorithms for BCBMs are based on guidelines for secondary brain metastases in general rather than being specific for breast cancer. These include algorithms for the use of corticosteroids to reduce peritumoral edema, which are based primarily on recommendations for the local treatment of CNS disease.[18] When a patient has a small number of tumors or a large tumor that is significantly compressing surrounding tissue, or when obtaining a tissue sample for diagnosis is critical, surgical resection and stereotactic radiosurgery (SRS) are usually considered. SRS is typically used in patients with surgically inaccessible metastases and in those who are not surgical candidates. Whole-brain radiotherapy (WBRT) is generally recommended when there are multiple lesions, particularly when the lesions are large. Multiple randomized trials have demonstrated improved intracranial control when WBRT is given following local approaches (ie, SRS and/or surgery),[19-21] although this approach is associated with a greater risk of a neurocognitive decline compared with SRS alone.[22] This is a concern particularly in patients who have a relatively longer survival time following the diagnosis of BCBMs, such as patients with HER2-positive disease.[5] Since WBRT has not demonstrated an overall survival (OS) benefit in the management of CNS metastases, a discussion of the risks and benefits is critical. Given the potential short- and long-term effects of WBRT, the development of systemic options that might delay the need for palliative WBRT is an important clinical need.
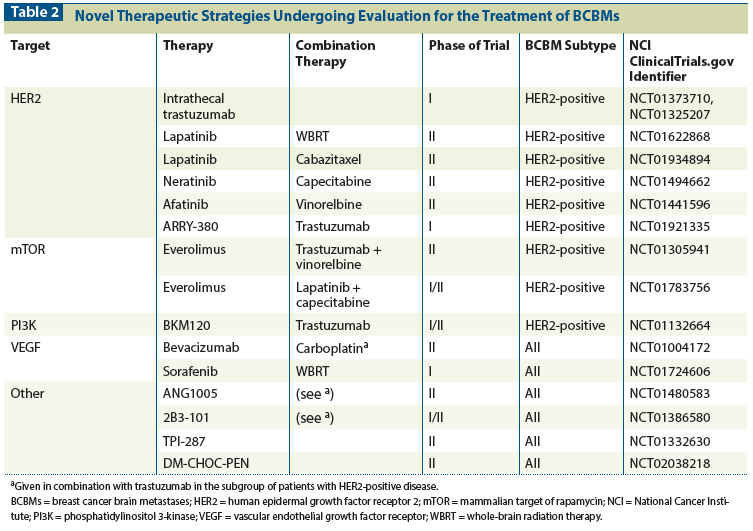
There are currently no approved systemic chemotherapy regimens for the management of BCBMs. The majority of the older trials included patients with primary tumor of multiple origins, and small subgroups with BCBMs. The results are further confounded by differences in prior chemotherapy and CNS radiation exposure. Traditional systemic chemotherapies included cisplatin, temozolomide, etoposide, capecitabine, epothilone B analogues, and various combinations of these agents. Except in the case of the platinum agents, the reported CNS objective response rates (ORRs) were typically modest and the duration of benefit was short (< 4 months).[23] Trials of the platinum agents, in which the response rate was higher, are limited in relevance by differences in the patient populations compared with those in the modern era. In particular, patients in those trials tended to be less heavily pretreated in either the adjuvant or metastatic setting. More recently, Anders and colleagues reported results of a phase II trial of irinotecan plus iniparib in pretreated patients with TNBC.[24] Iniparib is a drug initially developed as a poly(ADP- ribose) polymerase (PARP) inhibitor but subsequently shown not to have any PARP inhibitor activity.[25] Nevertheless, clinical activity was observed, with a CNS clinical benefit rate of 30%, albeit with a median overall time to progression of just over 2 months.[24] Given that irinotecan is known to have CNS activity in other tumor types (eg, glioblastoma), it is reasonable to postulate that most, if not all, of the activity observed in the trial was attributable to this agent. Further analyses are underway to identify factors predictive of response.
Currently, none of these agents are considered standard of care for first-line management of BCBMs, although they could be considered on a case-by-case basis, and in the setting of disease that has progressed through standard radiotherapy-based approaches. More recently, there has been a greater emphasis on evaluation of therapies in trials specific for BCBMs. Table 1 highlights current systemic therapies being investigated in this setting, including third-generation taxanes such as cabazitaxel, TPI-287, and ANG1005, in phase II trials of patients with BCBMs (National Cancer Institute ClinicalTrials.gov identifiers NCT01913067, NCT01332630, and NCT01480583 respectively). No investigations of these chemotherapy agents have yet been translated into routine clinical use.
In the setting of ER-positive BCBMs, key determinants of outcome from the time of CNS recurrence are the overexpression of the HER2 receptor and treatment with HER2-directed therapies. Median survival times from time of diagnosis of BCBMs in the ER-positive/HER2-negative and ER-positive/HER2-positive subsets were 10 months and 22.7 months, respectively, with the former outcome similar to that in the TNBC subset (median survival time, 7 months).[5] A likely explanation is that many of these patients have hormone-refractory disease by the time CNS metastases appear, therefore rendering this class of treatment of limited value when used alone.
HER2-Directed Therapies
The greatest inroads in systemic treatment of BCBMs have been made in patients with the HER2-positive breast cancer subtype, in keeping with the efficacy of HER2-directed therapies. Historically, it has been thought that the brain was a sanctuary site for trastuzumab, as well as for newer agents such as trastuzumab-emtansine (T-DM1) and pertuzumab, due to the relative difficulty of large monoclonal antibody therapies in penetrating the BBB.[12,26] Newer in vivo positron emission tomography (PET) imaging data in a limited number of patients using 89Zr-trastuzumab have demonstrated CNS uptake of trastuzumab into brain metastases, indicating that at least in some patients, trastuzumab can cross a disrupted BBB.[27] Interestingly, in light of these data, patients appear to derive a survival benefit with the continuation of trastuzumab after development of BCBMs.[28,29] More recently, a single case report was published describing a CNS response to T-DM1.[30] Furthermore, in a subset analysis of the EMILIA trial, which randomized patients with pretreated HER2-positive metastatic breast cancer either to lapatinib plus capecitabine or to T-DM1, patients with treated and stable brain metastases who were enrolled in the study fared better with T-DM1 in terms of overall survival.[31] Based on these data, trials to directly test the activity of T-DM1 in progressive HER2-positive BCBMs are currently being designed, as is a trial to test the role of high-dose trastuzumab. In addition, in the setting of leptomeningeal metastases, two clinical trials are underway (one in the US and one in France) which are testing intrathecal trastuzumab (ClinicalTrials.gov identifiers NCT01373710 and NCT01325207).
Another approach has been to consider small-molecule inhibitors, in place of large monoclonal antibodies. Lapatinib, a small-molecule tyrosine kinase inhibitor targeting the cytoplasmic ATP-binding sites of the kinase domains of HER2 and epidermal growth factor receptor (EGFR), has been developed as another systemic strategy for targeting the HER2 signaling pathway. We conducted two phase II studies with single-agent lapatinib in patients with HER2-positive BCBMs who progressed on trastuzumab therapy and prior WBRT. The CNS ORR was low in these studies (3% to 6%), and the addition of topotecan to lapatinib did not improve response rates.[32-34] However, the response rate was higher when lapatinib was given in combination with capecitabine, with a range of 18% to 38%.[34-36]
More recently, LANDSCAPE, a single-arm phase II study of lapatinib and capecitabine for patients with previously untreated HER2-positive BCBMs, reported a CNS ORR of 66%, a median time to CNS progression of 5.5 months, and a median time to WBRT of 8.3 months.[37] It is not surprising that response rates are higher in first-line treatment of CNS metastases in patients who have not received prior CNS-directed therapy. We believe this represents a viable alternative first-line treatment option for patients with HER2-positive BCBMs, particularly those with asymptomatic, low-volume disease, for whom local therapies such as radiotherapy have been the standard of care, and among whom historical rates of response to WBRT are reported at between 27% and 50%.[38-40] In light of the encouraging CNS responses seen with this combination regimen, other lapatinib chemotherapy combinations, such as lapatinib and the third-generation taxane cabazitaxel (ClinicalTrials.gov identifier NCT01934894), are now being evaluated in patients with BCBMs.
Lapatinib has also been evaluated as a radiosensitizer in combination with WBRT. In a phase I trial in patients with HER2-positive BCBMs, lapatinib was given at 750 mg bid on day 1, followed by dose levels of 1,000 mg, 1,250 mg, or 1,500 mg daily. WBRT (37.5 Gy over 15 fractions) commenced 1 to 8 days after treatment with lapatinib was initiated, followed with maintenance trastuzumab and lapatinib upon completion of WBRT.[41] Toxicity was an issue in this study, and it did not meet the predefined toxicity criteria. However, the CNS ORR by predefined volumetric criteria was 79%, which is higher than historical response rates observed with WBRT alone.[38-40] The limitation of the study was that it was a nonrandomized, single-arm trial, such that the contribution of lapatinib could not be assessed directly. Indeed, a similar approach has been evaluated with concurrent trastuzumab and WBRT, with the authors reporting a bidimensional response rate of 74% at 6 weeks.[42] To this end, a phase II trial jointly conducted by the Korean Radiation Oncology Group and the Radiation Therapy Oncology Group, in which patients are randomized to receive WBRT with or without lapatinib, is currently recruiting (ClinicalTrials.gov identifier NCT01622868). Given the number of HER2-directed therapies currently approved for use in patients with metastatic breast cancer (eg, trastuzumab, pertuzumab, lapatinib, and T-DM1), one important facet upon which novel HER2-directed agents can potentially distinguish themselves is CNS activity. To this end, the irreversible HER2 inhibitors neratinib and afatinib are under active investigation in the context of BCBMs (ClinicalTrials.gov identifiers NCT01494662 and NCT01441596, respectively). In addition, a number of other HER2 inhibitors are moving forward in this space, including ARRY-380 (ONT-380) and KD019. ARRY-380 is a HER2-selective inhibitor with minimal EGFR-inhibitory effect. Both the parent drug and metabolite cross the BBB to some degree, and have activity in intracranial tumor models. KD019 is a multitargeted kinase inhibitor of EGFR, HER2, and vascular endothelial growth factor (VEGF) receptor 2 (VEGFR-2). As will be discussed, anti-angiogenic approaches for the treatment of brain metastases may be of interest based on the limited data available to date.
Novel Therapies for BCBMs
The ideal systemic therapy for BCBMs should specifically target ligands that are expressed by tumor cells and are responsible for the tumorigenic phenotype, should adequately penetrate the BBB, should effectively control extracranial disease, and should be relatively well tolerated. Although a therapy that is designed specifically for BCBMs and fulfills all these criteria has not yet been developed, the majority of novel approaches to therapy for BCBMs build on promising efficacy demonstrated in the context of extracranial metastasis.
Trial design is a major consideration in the evaluation of novel therapies for management of BCBMs. There has been significant progress in the establishment of standardized guidelines to assess CNS response, progression, neurocognitive function, and quality of life.[43,44] However, most novel agents evaluated for treatment of BCBMs are being assessed in the setting of disease that is refractory to systemic therapy, and most often in patients who have received local therapies such as radiation. The majority of studies of BCBMs include small patient cohorts and lack a control arm, as there are no systemic therapies approved for use in this setting. The option of evaluating a specific systemic therapy prior to WBRT, and the potential benefits of doing so, are discussed earlier in the context of the LANDSCAPE trial.[38] Table 2 describes several novel therapies currently under investigation for BCBMs.
PI3K pathway–directed therapy
There has been much interest in therapeutics targeting the phosphatidylinositol 3-kinase (PI3K)–mammalian target of rapamycin (mTOR) pathway, where activating mutations of PIK3CA and/or loss of PTEN expression are among the most common genetically altered pathways in breast cancer; PIK3CA mutations are found in about a third of HER2-positive breast cancers, and PTEN loss is observed in about half of TNBC cases.[45] Aberrations in this signaling pathway have also been demonstrated in the majority of BCBMs.[46] Importantly, PI3K-mTOR activation is upregulated in resistance to HER2-directed therapies.[47,48] The plethora of drugs targeting this pathway that are now in clinical development include mTOR, PI3K, and dual mTOR/PI3K inhibitors. For two of these drugs (everolimus and BKM120), there are currently trials specifically recruiting patients with BCBMs, and it is likely that more relevant trials will follow.
The oral rapamycin analog and mTOR inhibitor everolimus is able to cross the BBB. The seminal trial for this therapy in the ER-positive breast cancer subtype is BOLERO-2, a phase III study comparing the steroidal aromatase inhibitor exemestane, with or without everolimus, in patients with advanced ER-positive disease who had progressed on a nonsteroidal aromatase inhibitor. The addition of everolimus improved median progression-free survival (PFS) from 4.1 months to 10.6 months (hazard ratio = 0.36).[49] These results have led to US Food and Drug Administration approval for use of everolimus in this setting. In patients with advanced HER2-positive disease, the phase III BOLERO-3 trial compared the efficacy of vinorelbine and trastuzumab with and without everolimus. The addition of everolimus improved PFS by 22%.[50] Patients with BCBMs were excluded from both the BOLERO-2 and BOLERO-3 trials, however. Building on these promising data, everolimus is now being evaluated in combination with lapatinib and capecitabine in a phase Ib/II trial in patients with HER2-positive BCBMs (ClinicalTrials.gov identifier NCT01783756). In a similar group of patients, everolimus is also being evaluated in combination with trastuzumab and vinorelbine, in single-arm phase II study (ClinicalTrials.gov identifier NCT01305941).BKM120 is an oral, pan-PI3K inhibitor that penetrates the BBB.[51] A phase I/II study of the combination of trastuzumab and BKM120 in patients who have relapsed on trastuzumab is underway, with an expansion cohort in patients with HER2-positive BCBMs (ClinicalTrials.gov identifier NCT01132664).
VEGF inhibitors
The general enthusiasm for VEGF inhibitors in breast cancer was dampened by meta-analyses that failed to demonstrate an OS benefit in the metastatic setting, primarily in HER2-negative breast cancer. However, VEGF inhibitors have continued to play a role in the treatment of refractory glioblastoma. Because of concern about CNS hemorrhage, most of the randomized studies of breast cancer excluded patients with a history of brain metastases, and none permitted enrollment of patients with active CNS disease.
We recently reported on a phase II trial of carboplatin and bevacizumab in patients with BCBMs. The majority of patients in this trial had received prior brain irradiation and had HER2-positive disease, with most having been treated previously with HER2-directed therapy. The primary endpoint was CNS ORR in patients with progressive BCBMs.[52] We reported a CNS ORR of 63% by prespecified volumetric criteria. The CNS ORR by Response Evaluation Criteria in Solid Tumors (RECIST) was 45%, and the median number of cycles of therapy received was 8, suggesting that this combination is associated with a high rate of durable responses. Another trial in patients with BCBMs reported a CNS ORR of 60% with the combination of bevacizumab, etoposide, and cisplatin in patients who had CNS progression following prior WBRT.[53] However, because of the potential effect of bevacizumab on vascular permeability, one caveat is that possibly the responses were simply an effect of reduced gadolinium leakiness, and thus less contrast enhancement, as opposed to true tumor regressions. Randomized trials with endpoints other than response will be required to determine the true contribution of bevacizumab to clinical outcomes in patients.
PARP inhibitors
PARP inhibitors disrupt DNA repair and have been developed for treatment of breast and ovarian cancer. This class of drugs has been found to be particularly effective in BRCA1 and BRCA2 mutation–associated breast and ovarian cancer, and is currently being evaluated for use in sporadic TNBC and ovarian cancer.[54] A high incidence of BCBMs has been observed in patients carrying BRCA mutations.[55] A phase I trial of ABT-888 with WBRT has recently been completed, and we await results of its tolerability profile (ClinicalTrials.gov identifier NCT00649207).
Summary and Conclusion
BCBMs are common in patients with advanced breast cancer, occurring in half of patients with HER2-positive disease or TNBC. The breast cancer subtype is a major determinant of the course of the disease. Patients with HER2-positive metastatic breast cancer who receive effective HER2-directed therapies have had the natural history of their disease altered, with great improvements in the control of systemic disease; however, this has resulted in the growing problem of an increasing cumulative incidence of CNS events, and the need for multiple lines of CNS-directed therapy. Development of new systemic therapies for breast cancer-coupled with improvements in trial design, in imaging modalities, and in the definition and measurement of clinical endpoints-has led to a renewed interest in developing novel therapeutic approaches for BCBMs. Despite the increasing number of trials of systemic therapies specific for BCBMs, however, local therapy options remain the current standard of care for these patients. The challenge ahead is to move some of the promising therapies from early-phase trials into a randomized phase II or III setting, to advance the standard of care for patients with BCBMs. To decrease the heterogeneity of responses, specific consideration will need to be given to the specific breast cancer subtype and to the identification of novel predictive biomarkers of response.
Financial Disclosure:Dr. Lin receives clinical trial funding from Array Biopharma, Genentech, GlaxoSmithKline, and Novartis. Dr. Lim has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608-17.
2. Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271-7.
3. Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:2111-7.
4. Berghoff A, Bago-Horvath Z, De Vries C, et al. Brain metastases-free survival differs between breast cancer subtypes. Br J Cancer. 2012;106:440-6.
5. Sperduto PW, Kased N, Roberge D, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. 2013;112:467-72.
6. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274-84.
7. Polli JW, Olson KL, Chism JP, et al. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab Dispos. 2009;37:439-42.
8. Pitz MW, Desai A, Grossman SA, Blakeley JO. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104:629-38.
9. Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013;14:244-8.
10. Goss PE, Smith IE, O’Shaughnessy J, et al. Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:88-96.
11. Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834-43.
12. Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972-7.
13. Olson EM, Abdel-Rasoul M, Maly J, et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24:1526-33.
14. Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638-45.
15. Lee LJ, Alexander B, Schnitt SJ, et al. Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer. 2011;117:3093-100.
16. Dawood S, Shaikh AJ, Buchholz TA, et al. The use of bevacizumab among women with metastatic breast cancer: a survey on clinical practice and the ongoing controversy. Cancer. 2012;118:2780-6.
17. Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242-8.
18. Kalkanis SN, Linskey ME. Evidence-based clinical practice parameter guidelines for the treatment of patients with metastatic brain tumors: introduction. J Neurooncol. 2010;96:7-10.
19. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134-41.
20. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
21. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-91.
22. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037-44.
23. Lim E, Lin NU. New insights and emerging therapies for breast cancer brain metastases. Oncology (Williston Park). 2012;26:652-9, 63.
24. Anders CK, Deal AM, Abramson VG, et al. TBCRC 018: phase II study of iniparib plus chemotherapy to treat triple-negative breast cancer (TNBC) central nervous system (CNS) metastases (mets). J Clin Oncol. 2013;31(suppl):abstr 515.
25. Sinha G. Downfall of iniparib: a PARP inhibitor that doesn’t inhibit PARP after all. J Natl Cancer Inst. 2014;106:djt447.
26. Burstein HJ, Lieberman G, Slamon DJ, et al. Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol. 2005;16:1772-7.
27. Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87:586-92.
28. Park YH, Park MJ, Ji SH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer. 2009;100:894-900.
29. Karam I, Hamilton S, Nichol A, et al. Population-based outcomes after brain radiotherapy in patients with brain metastases from breast cancer in the pre-trastuzumab and trastuzumab eras. Radiat Oncol. 2013;8:12.
30. Bartsch R, Berghoff AS, Preusser M. Breast cancer brain metastases responding to primary systemic therapy with T-DM1. J Neurooncol. 2014;116:205-6.
31. Krop I, Lin NU, Blackwell K, et al. Efficacy and safety of trastuzumab emtansine (T-DM1) vs lapatinib plus capecitabine (XL) in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC) and central nervous system (CNS) metastases: results from a retrospective exploratory analysis of EMILIA. Cancer Research. 2013;73:P4-12-27.
32. Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993-9.
33. Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452-9.
34. Lin NU, Eierman W, Greil R, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol. 2011;105:613-20.
35. Metro G, Foglietta J, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22:625-30.
36. Sutherland S, Ashley S, Miles D, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases-the UK experience. Br J Cancer. 2010;102:995-1002.
37. Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14:64-71.
38. Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486-93.
39. Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2012;4:CD003869.
40. Broadbent AM, Hruby G, Tin MM, et al. Survival following whole brain radiation treatment for cerebral metastases: an audit of 474 patients. Radiother Oncol. 2004;71:259-65.
41. Lin NU, Freedman RA, Ramakrishna N, et al. A phase I study of lapatinib with whole brain radiotherapy in patients with Human Epidermal Growth Factor Receptor 2 (HER2)-positive breast cancer brain metastases. Breast Cancer Res Treat. 2013;142:405-14.
42. Chargari C, Idrissi HR, Pierga JY, et al. Preliminary results of whole brain radiotherapy with concurrent trastuzumab for treatment of brain metastases in breast cancer patients. Int J Radiat Oncol Biol Phys. 2011;81:631-6.
43. Lin NU, Lee EQ, Aoyama H, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol. 2013;14:e396-406.
44. Lin NU, Wefel JS, Lee EQ, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013;14:e407-16.
45. Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011;10:1093-101.
46. Adamo B, Deal AM, Burrows E, et al. Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Res. 2011;13:R125.
47. Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395-402.
48. Jegg AM, Ward TM, Iorns E, et al. PI3K independent activation of mTORC1 as a target in lapatinib-resistant ERBB2+ breast cancer cells. Breast Cancer Res Treat. 2012;136:683-92.
49. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520-9.
50. O’Regan R, Ozguroglu M, Andre F, et al. Phase III, randomized, double-blind, placebo-controlled multicenter trial of daily everolimus plus weekly trastuzumab and vinorelbine in trastuzumab-resistant, advanced breast cancer (BOLERO-3). J Clin Oncol. 2013;31(suppl):abstr 505.
51. Wen PY, Yung WKA, Mellinghoff IK, et al. Phase II trial of the phosphatidyinositol-3 kinase inhibitor BKM120 in recurrent glioblastoma. J Clin Oncol. 2013;31(suppl):abstr 2015.
52. Lin NU, Gelman RS, Younger WJ, et al. Phase II trial of carboplatin and bevacizumab in patients with breast cancer brain metastases. 2013;31(suppl):abstr 513.
53. Lu YS, Chen WW, Lin CH, et al. Bevacizumab, etoposide, and cisplatin (BEEP) in brain metastases of breast cancer progressing from radiotherapy: results of the first stage of a multicenter phase II study. J Clin Oncol. 2012;30(suppl):abstr 1079.
54. Balmana J, Domchek SM, Tutt A, Garber JE. Stumbling blocks on the path to personalized medicine in breast cancer: the case of PARP inhibitors for BRCA1/2-associated cancers. Cancer Discov. 2011;1:29-34.
55. Tischkowitz MD, Foulkes WD. The basal phenotype of BRCA1-related breast cancer: past, present and future. Cell Cycle. 2006;5:963-7.
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.