Metastatic Adrenocortical Carcinoma With a Prolonged Response to Mitotane
Adrenocortical carcinoma is a rare disease, with an annual incidence rate ranging from 0.5 to 2.0 cases per million individuals.
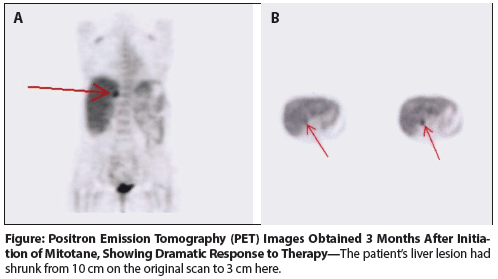
Figure: Positron Emission Tomography (PET) Images Obtained 3 Months After Initiation of Mitotane, Showing Dramatic Response to Therapy
The Case:In 2002, a 55-year-old woman presented to medical attention with cough and wheezing and was treated for presumed pneumonia. She had a history of breast cancer successfully treated with lumpectomy, radiotherapy, and adjuvant chemotherapy, as well as bilateral papillary thyroid cancer that was treated with thyroidectomy and adjuvant radioactive iodine. The workup included a CT scan that revealed a large left renal lesion; a 10-cm lesion in the left lobe of the liver; and multiple bilateral pulmonary lesions, including a 3-cm lesion in the right lower lobe-all presumed to be metastatic renal cell carcinoma. Cytoreductive nephrectomy with adrenalectomy was performed. Pathology results revealed a 16-cm nonfunctioning adrenocortical carcinoma. An intraoperative biopsy of the liver lesion confirmed metastatic disease in the liver.
The patient was initially treated with mitotane and replacement hydrocortisone at a dose of 1 g orally 4 times daily, with a plan to increase the dose to maximum tolerability. Side effects were low-grade and included nausea, diarrhea, fatigue, and dizziness. The dose of mitotane was eventually increased to as much as 10 g daily. However, this dose was poorly tolerated; the patient ultimately received a stable regimen of 6–8 g daily.
A positron emission tomography (PET)/CT scan performed 3 months after the initiation of mitotane therapy demonstrated that the liver lesion had decreased to 3 cm (Figure). A PET/CT scan 1 year after mitotane had been started showed resolution of all pulmonary nodules and a stable 3-cm liver lesion. Nearly 2 years after the original surgery, laparoscopic radiofrequency ablation was performed to address the persistent hepatic lesion. Mitotane was discontinued 3 years after initiation, given the absence of residual disease on scans. After a nearly 2-year hiatus from mitotane, repeat imaging showed an isolated 1-cm pulmonary lesion in the right upper lobe. A wedge resection was performed; pathologic examination confirmed this lesion to be recurrent adrenocortical carcinoma. Therapy with mitotane was resumed and again dosed to maximum tolerability.
The patient survived for an additional 7 years and was maintained on mitotane without treatment interruption and with minimal side effects, during which time she lived an entirely normal life. She was followed closely with repeat PET/CT imaging on an every-6-months basis and consistently showed no evidence of recurrence. Her last scans were obtained in early 2014, just prior to her sudden death from natural causes unrelated to her malignancy, 12 years after the original diagnosis of widely metastatic disease.
Discussion
Adrenocortical carcinoma is a rare disease, with an annual incidence rate ranging from 0.5 to 2.0 cases per million individuals.[1] The median age at diagnosis is about 55 years, with approximately 21% of patients presenting with metastatic disease.[2] The extreme rarity of this cancer has precluded extensive investigation and the development of active treatments.
Several clinicopathologic characteristics have been linked to prognosis and outcome. Else et al compiled an institutional series of 391 patients with adrenocortical carcinoma of various stages.[3] As expected, increasing stage and tumor grade were associated with poorer survival, as was the level of cortisol production (note that in the case described here, the carcinoma was nonfunctioning). Recently, it has been suggested that adrenocortical carcinoma may be a Lynch syndrome–associated cancer.[4] Ultimately, though, only 3 of 114 patients (3.2%) were found to have family histories suggestive of Lynch syndrome; this frequency was similar to that seen in colorectal and endometrial cancers.
Assié et al[5] performed an assessment of gene alterations present in adrenocortical carcinomas and reported whole exome sequencing in a series of more than 100 patients.[6] Alterations in several known driver genes, including RB1, MEN1, CDKN2A, and TP53 were observed. There were also four previously uncharacterized alterations, which were noted in ZNRF3, DAXX, TERT, and MED12. ZNRF3, a ubiquitin ligase, was found to be altered in 21% of patients. Two separate genomic profiles with distinct clinical outcomes were defined in this study. Activation of β-catenin, which results in activation of the Wnt/β-catenin pathway, has been associated with decreased overall survival (OS),[6,7] as has high expression of steroidogenic factor 1.[8,9] Other prognostic markers have also been proposed-for example, Heinze et al characterized polymorphisms in the TP53 gene using blood collected from 72 patients.[10] Several infrequent single-nucleotide polymorphisms that corresponded with a poor prognosis were identified. However, we do not know what biomarkers were expressed in the tumor of our patient.
For more than 50 years, mitotane (o,p'-DDD, a chlorinated hydrocarbon related to dichlorodiphenyltrichloroethane [DDT]) has been the standard of care for adrenocortical carcinoma in both the adjuvant and metastatic settings. In the adjuvant setting, recommendations were predicated on a retrospective analysis of 177 patients with localized adrenocortical carcinoma treated at German and Italian cancer centers.[11] On multivariate analysis, the use of mitotane was associated with an improvement in recurrence-free survival. Unfortunately, in the metastatic disease setting, single-agent mitotane has rarely produced response rates greater than 20% in any trial,[12] and no prolongation of OS has ever been demonstrated.
In order to improve treatment of adrenocortical carcinoma, a phase III trial was performed, in which 304 patients with advanced disease were randomized to receive either mitotane in combination with etoposide, doxorubicin, and cisplatin (EDP) or mitotane with streptozotocin.[13] The response rate was higher in patients receiving mitotane plus EDP (23.2% vs 9.2%; P < .001). Furthermore, progression-free survival (PFS) was also prolonged (5.0 months vs 2.1 months; P < .001). However, no differences in OS were observed. Guidelines from the European Society for Medical Oncology suggest that patients with low-volume metastatic disease be treated with mitotane alone.[14] In contrast, mitotane plus EDP is recommended for patients with a high tumor burden or prior exposure to mitotane.
Because of the generally dismal results associated with mitotane treatment, attempts have been made in small numbers of patients to characterize the activity of other therapies. For example, in a phase II study of cisplatin plus docetaxel in 19 patients, the response rate was 21%, with a median PFS of 3 months.[15] The median OS was 12.5 months. Targeted therapeutics have been assessed in advanced adrenocortical carcinoma, but only minimal activity was observed with sunitinib. In a phase II study of 35 patients with advanced disease, the observed response rate was 15.4%, with a PFS of 2.8 months.[16]
How can we explain the long, almost miraculous, duration of response in our patient to single-agent mitotane, a drug not associated with particularly impressive activity? One study (N = 91) provides evidence that if the level of mitotane (in combination with cytotoxic chemotherapy) in the plasma is greater than 14 mg/mL, OS may be prolonged (24 months vs 18 months; hazard ratio [HR] = 0.52; P = .04).[17] The benefit was enhanced in patients (n = 27) treated with mitotane as monotherapy (119 months vs 18 months; HR = 0.26; P = .02).[17] Unfortunately, most patients cannot tolerate plasma drug levels this high[18]; also, the number of patients evaluated was small, and the data were retrospective. Moreover, the absorption of mitotane, because of its lipophilicity, is highly variable, making the attainment of prespecified plasma levels challenging.
In our patient’s case, mitotane monotherapy appeared to produce a remission until we allowed a treatment holiday. Furthermore, after surgical resection of a recurrence, the patient enjoyed the remaining 7 years of her life apparently cancer-free as a result of restarting mitotane. While mitotane levels were never determined in this patient, her subsequent prolonged course after aggressive treatment suggests that treating her with mitotane to maximum tolerability was the correct decision, as we believe that she achieved one of the longest responses to mitotane on record.
Financial Disclosure: Dr. Olsson reseives research support from Advantagene, Inc, Dendreon Corp, Exosome Diagnostics, Inc, and Medivation, Inc; he is part owner of a patent used by Dianon, and he owns stock in Egonix. The other authors have no significant financial interest in or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Kerkhofs TM, Verhoeven RH, Bonjer HJ, et al. Surgery for adrenocortical carcinoma in The Netherlands: analysis of the National Cancer Registry data. Eur J Endocrinol. 2013;169:83-9.
2. Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130-6.
3. Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev. 2014;35:282-326.
4. Raymond VM, Everett JN, Furtado LV, et al. Adrenocortical carcinoma is a Lynch syndrome-associated cancer. J Clin Oncol. 2013;31:3012-8.
5. Assié G, Letouzé E, Fassnacht M, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46:607-12.
6. Bonnet S, Gaujoux S, Launay P, et al. Wnt/β-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab. 2011;96:E419-26.
7. Heaton JH, Wood MA, Kim AC, et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am J Pathol. 2012;181:1017-33.
8. Sbiera S, Schmull S, Assié G, et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J Clin Endocrinol Metab. 2010;95:E161-71.
9. Duregon E, Volante M, Giorcelli J, et al. Diagnostic and prognostic role of steroidogenic factor 1 in adrenocortical carcinoma: a validation study focusing on clinical and pathologic correlates. Hum Pathol. 2013;44:822-8.
10. Heinze B, Herrmann LJ, Fassnacht M, et al. Less common genotype variants of TP53 polymorphisms are associated with poor outcome in adult patients with adrenocortical carcinoma. Eur J Endocrinol. 2014;170:707-17.
11. Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372-80.
12. De Francia S, Ardito A, Daffara F, et al. Mitotane treatment for adrenocortical carcinoma: an overview. Minerva Endocrinol. 2012;37:9-23.
13. Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189-97.
14. Berruti A, Baudin E, Gelderblom H, et al. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii131-8.
15. Urup T, Pawlak WZ, Petersen PM, et al. Treatment with docetaxel and cisplatin in advanced adrenocortical carcinoma, a phase II study. Br J Cancer. 2013;108:1994-7.
16. Kroiss M, Quinkler M, Johanssen S, et al. Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab. 2012;97:3495-503.
17. Hermsen IG, Fassnacht M, Terzolo M, et al. Plasma concentrations of o,p'DDD, o,p'DDA, and o,p'DDE as predictors of tumor response to mitotane in adrenocortical carcinoma: results of a retrospective ENS@T multicenter study. J Clin Endocrinol Metab. 2011;96:1844-51.
18. Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:4551-64..