The Variegated Morphology of HPV-Related Neoplasms of the Penis
Our commentary aims to expand on the evolution and present state of the art in the pathology of HPV in penile cancer and precancerous lesions.
Oncology (Williston Park). 30(3):253–256.

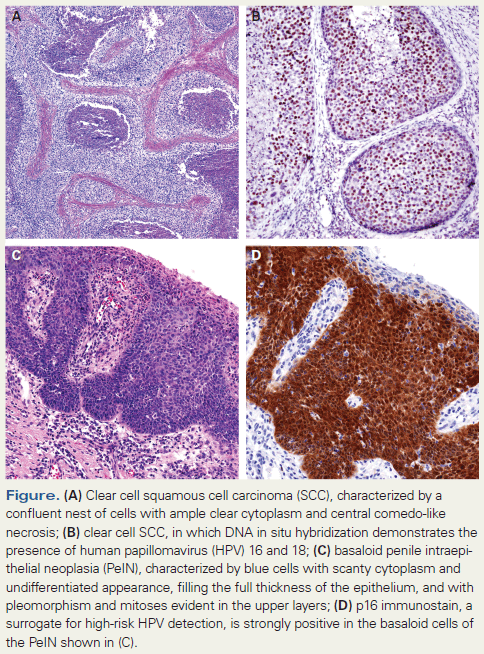
Table 1. 2016 WHO Pathologic Classification of Penile SCC According to HPV Presence
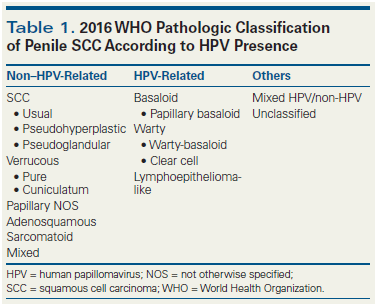
Table 2. 2016 WHO Penile Intraepithelial Neoplasia (PeIN) Subtypes
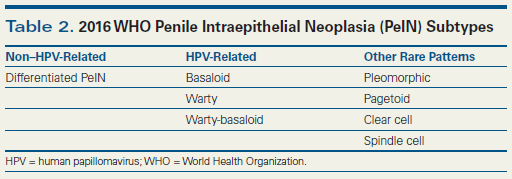
Figure
We have read with great interest the review by Drs. Stratton and Culkin covering the various levels of human papillomavirus (HPV) research in penile cancer, including epidemiologic, clinical, pathologic, and molecular domains.[1] Our commentary aims to expand on the evolution and present state of the art in the pathology of HPV in penile cancer and precancerous lesions.
Since the recognition in 1995 of a relationship between subtypes of squamous cell carcinoma (SCC) and HPV,[2] the bimodal hypothesis of penile cancer pathogenesis-ie, that there are both HPV- and non–HPV-related etiologies-appears to be well established.[3] In fact, for the first time, a pathologic classification of penile cancer that has worldwide impact-such as the one proposed by the World Health Organization (WHO)-is based on these ideas (Table 1).[4] However, the interest in HPV has resulted in many studies that overlook the pathogenesis of the most common types of penile cancers, which are non–HPV-related. These squamous tumors are mostly characterized by maturation and gradual keratinization, and comprise a variety of architectural patterns designated as usual, verrucous, cuniculatum, pseudohyperplastic, papillary not otherwise specified (NOS), and pseudoglandular.[5] There is no explanation as to why some penile tumors are etiologically related to HPV and others are not. We performed an epidemiologic prospective study of 103 patients with penile cancer and classified their tumors, did whole-tissue-section (WTS) polymerase chain reaction (PCR) testing, and compared known risk factors in patients with HPV-positive and -negative tumors. Patients with HPV-related penile tumors had a past history of genital warts and more lifetime sexual partners than those patients with non–HPV-related cancers.[6] We also found a preferential relationship between certain subtypes of SCC and the presence of HPV in penile cancer, which has been a major area of interest for us over the last 15 years.[7-10]
HPV in Subtypes of SCC
Since our first HPV detection study of 117 penile cancer cases, in which we identified a preferential association of the virus with basaloid and condylomatous (warty) carcinomas,[2] we have examined about 1,300 cases of invasive penile carcinomas in the search for other types that may be related to HPV. The first group of tumors identified were HPV-positive usual carcinomas mixed with either warty or basaloid carcinoma. Despite the distinctive appearances of warty and basaloid carcinomas, some mixed tumors were entirely composed of warty and basaloid carcinoma, with an exophytic condylomatous surface and deeply invasive basaloid tumor components. We designated these tumors as warty-basaloid carcinomas, reflecting their morphologic makeup.[11] Another tumor that did not fit into either the warty or classical basaloid carcinoma classification, but which had a basaloid cell type with an exophytic surface outgrowth, was the papillary variant of basaloid carcinoma.[12] This tumor had to be separated from the morphologically distinct and etiologically different papillary NOS variant of SCC, also with an exophytic pattern but without condylomatous features and with HPV and p16 negativity.[13] Another unusual tumor, originally thought to be metastatic from the kidney, is the clear cell carcinoma primary in the penis. This is an aggressive tumor composed almost entirely of clear cells, but with a nesting growth pattern and comedonecrosis similar to what is seen in basaloid carcinomas (Figure A and B). Cytologically, these cells resemble warty carcinoma clear cells but lack the exophytic growth of warty carcinomas.[14] All of these neoplasms share a common characteristic: they are composed of either basaloid or warty-like cells in specific architectural configurations and are relatively easy to recognize. We also found a group of tumors which, when properly diagnosed, are consistently negative for HPV: these include verrucous, papillary NOS, pseudoglandular, pseudohyperplastic, and sarcomatoid carcinomas. Except for the latter, these neoplasms are keratinizing, mature, low-grade variants of penile SCC. It was puzzling to find some HPV-positive cases in the usual SCC category, since the prevalence of the virus in this tumor type is much lower than in classic HPV-related tumors. In an effort to discover more about these observations, and being fortunate enough to be collaborators on a survey of 1,000 patients from five continents,[15] we reviewed 60 (6%) HPV-positive tumors originally classified as usual SCC. There were only 26 (2.6 %) bona fide usual SCCs. The other HPV-positive tumors were represented by mixed usual warty/basaloid patterns and a variety of poorly differentiated tumors with unusual morphologic features, including solid, trabecular, small cell, medullary, lymphoepithelioma-like, and Hodgkin-like with marked eosinophilia. Among these unusual HPV-positive SCCs, there are two new distinct types-lymphoepithelioma-like carcinomas and medullary carcinomas-which are the most recently described HPV-related variants of penile SCCs.[16,17] These tumors, composed of larger poorly differentiated cells, are morphologically and cytologically different from those harboring basaloid or condylomatous cells. This leads us to conclude that HPV is very rare in usual penile SCC and that HPV-positive tumors are morphologically distinctive. It is now possible to identify HPV-positive tumors by observing the histology with hematoxylin and eosin staining, and p16 stain can also be used to distinguish between HPV-positive tumors and non–HPV-related tumors in difficult cases. The significance of separating HPV-related neoplasms into distinct morphologic types will not be well known until definitive molecular analyses are performed. The extremes of the spectrum, such as the condylomatous or warty carcinomas, which have an excellent prognosis, and the basaloid carcinoma, with a poorer prognosis, will justify this effort. Patients with HPV-related neoplasms at other sites carry a better prognosis than those with non–HPV-related tumors, but in penile cancer the studies are contradictory.[18,19]
Penile Intraepithelial Neoplasia (PeIN) Subtypes and HPV Genotypes
Geographic differences in the incidence of invasive penile cancer is well known: there are about 2 to 4 cases per 100,000 inhabitants in Asia, Africa, and Latin America, and 1 case per 100,000 in countries in North America and Europe. However, after examining cases from many countries, we found no differences in the distribution of subtypes of invasive carcinomas.[20] At the precancerous level, though, we found a higher prevalence of HPV-related subtypes of PeIN in Paris than in cases diagnosed in Paraguay, where differentiated PeIN (non–HPV-related) was the most common precancerous lesion.[15,21]
The new WHO classification of PeIN separates HPV-related from non–HPV-related precancerous lesions (Table 2)[4,22]: differentiated PeINs are usually HPV-negative warty, and basaloid PeINs are usually HPV-positive (Figure C and D). There are few studies of the genotypic composition of PeIN,[23,24] and similar to studies of invasive carcinomas, the methodology used in these studies has usually been WTS-PCR. Recently, we demonstrated that laser capture microdissection (LCM)-PCR is a better precision instrument than WTS-PCR, because lesions in which WTS-PCR showed just one genotype were found to actually have multiple HPV genotypes when the lesion was carefully dissected with the laser instrument.[25] Unlike invasive penile carcinomas, where few HPV genotypes are usually identified, many more genotypes exist in basaloid and warty PeINs. In 115 lesions from 43 patients, we found 18 different HPV genotypes, including both high- and low-risk types. One interesting finding was that HPV 16 was common in basaloid PeIN but rare in warty PeIN (70% vs 20%). Warty PeIN was found to have a heterogeneous array of multiple and variable HPV genotypes, unlike basaloid PeIN.[26] These findings strongly support the approach used[4] to classify HPV-related and non–HPV-related PeIN and in subtyping variants of PeIN.
Are Common Condylomas Cancer Precursors?
Clinical warts are common in young males and frequently reported as a risk factor for developing cancer; however, the mechanisms of this relationship remain obscure. The predominant thinking is that all condylomas are benign, exophytic, wart-like lesions with koilocytosis and related to infection with low-risk HPV strains, such as HPV 6 and HPV 11. These lesions are not considered precancerous, according to older or current classification schemes. However, emerging data indicate that some condylomas show cytologic atypias and harbor high-risk HPV.[27-29] In a recent unpublished study, we found that 20% of otherwise exophytic or acuminate condylomas associated with penile neoplasia showed focal cytologic atypias and harbored high-risk HPV, suggesting they were likely precancerous. More studies should be conducted comparing large numbers of common condylomas in younger patients with those associated with neoplasia in older patients. Another interesting finding in the study just mentioned was the presence of HPV-positive flat lesions with uncharacteristic morphologic appearances, which varied from hyperplasia-like, to acanthotic thickening with mild atypias, to flat lesions with superficial minimal koilocytosis. These unusual subclinical lesions were located near PeIN or invasive carcinomas, and many of them contained high-risk HPV similar to the virus types found in adjacent neoplasia. It may be that these lesions were flat condylomas or similar to the ones reported by Bleeker and Meijer[28,29] under the name of “acetowhite lesions” in male partners of women with cervical intraepithelial neoplasia. Another explanation may be that these lesions are, like atypical condylomas, a missing link between common condylomas, PeIN, and invasive cancer.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Stratton KL, Culkin DJ. A contemporary review of HPV and penile cancer. Oncology (Williston Park). 2016;30:245-9, 252.
2. Gregoire L, Cubilla AL, Reuter VE, et al. Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst. 1995;87:1705-9.
3. Rubin MA, Kleter B, Zhou M, et al. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159:1211-8.
4. Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO classification of tumours of the urinary system and male genital organs. 4th ed. Lyon: IARC Press; 2015. [In Press]
5. Sanchez DF, Canete S, Fernandez-Nestosa MJ, et al. HPV- and non-HPV-related subtypes of penile squamous cell carcinoma (SCC): Morphological features and differential diagnosis according to the new WHO classification (2015). Semin Diagn Pathol. 2015;32:198-221.
6. Chaux A, Netto GJ, Rodriguez IM, et al. Epidemiologic profile, sexual history, pathologic features, and human papillomavirus status of 103 patients with penile carcinoma. World J Urol. 2013;31:861-7.
7. Chaux A, Cubilla AL, Haffner MC, et al. Combining routine morphology, p16(INK4a) immunohistochemistry, and in situ hybridization for the detection of human papillomavirus infection in penile carcinomas: a tissue microarray study using classifier performance analyses. Urol Oncol. 2014;32:171-7.
8. Chaux A, Pfannl R, Lloveras B, et al. Distinctive association of p16INK4a overexpression with penile intraepithelial neoplasia depicting warty and/or basaloid features: a study of 141 cases evaluating a new nomenclature. Am J Surg Pathol. 2010;34:385-92.
9. Cubilla AL, Lloveras B, Alejo M, et al. Value of p16(INK)(4)(a) in the pathology of invasive penile squamous cell carcinomas: A report of 202 cases. Am J Surg Pathol. 2011;35:253-61.
10. Cubilla AL, Lloveras B, Alejo M, et al. The basaloid cell is the best tissue marker for human papillomavirus in invasive penile squamous cell carcinoma: a study of 202 cases from Paraguay. Am J Surg Pathol. 2010;34:104-14.
11. Chaux A, Tamboli P, Ayala A, et al. Warty-basaloid carcinoma: clinicopathological features of a distinctive penile neoplasm. Report of 45 cases. Mod Pathol. 2010;23:896-904.
12. Cubilla AL, Lloveras B, Alemany L, et al. Basaloid squamous cell carcinoma of the penis with papillary features: a clinicopathologic study of 12 cases. Am J Surg Pathol. 2012;36:869-75.
13. Chaux A, Soares F, Rodriguez I, et al. Papillary squamous cell carcinoma, not otherwise specified (NOS) of the penis: clinicopathologic features, differential diagnosis, and outcome of 35 cases. Am J Surg Pathol. 2010;34:223-30.
14. Sanchez DF, Rodriguez IM, Piris A, et al. Clear cell carcinoma of the penis: an HPV-related variant of squamous cell carcinoma: a report of 3 cases. Am J Surg Pathol. 2016 Feb 4. [Epub ahead of print]
15. Alemany L, Cubilla A, Halec G, et al. Role of human papillomavirus in penile carcinomas worldwide. Eur Urol. 2016 Jan 4. [Epub ahead of print]
16. Mentrikoski MJ, Frierson HF Jr, Stelow EB, Cathro HP. Lymphoepithelioma-like carcinoma of the penis: association with human papilloma virus infection. Histopathology. 2014;64:312-5.
17. Cañete S, Clavero O, Sanchez DF, et al. Poorly differentiated solid (medullary) carcinoma: a distinctive HPV-related penile neoplasm. A report of 12 cases. Mod Pathol. 2016. [In Press]
18. Bezerra SM, Chaux A, Ball MW, et al. Human papillomavirus infection and immunohistochemical p16(INK4a) expression as predictors of outcome in penile squamous cell carcinomas. Hum Pathol. 2015;46:532-40.
19. Djajadiningrat RS, Jordanova ES, Kroon BK, et al. Human papillomavirus prevalence in invasive penile cancer and association with clinical outcome. J Urol. 2015;193:526-31.
20. Chaux A, Lezcano C, Cubilla AL, et al. Comparison of subtypes of penile squamous cell carcinoma from high and low incidence geographical regions. Int J Surg Pathol. 2010;18:268-77.
21. Soskin A, Vieillefond A, Carlotti A, et al. Warty/basaloid penile intraepithelial neoplasia is more prevalent than differentiated penile intraepithelial neoplasia in nonendemic regions for penile cancer when compared with endemic areas: a comparative study between pathologic series from Paris and Paraguay. Hum Pathol. 2012;43:190-6.
22. Epstein JI, Cubilla AL, Humphrey PA. Tumors of the prostate gland, seminal vesicles, penis, and scrotum. Silverberg SG, editor. Washington, DC: American Registry of Pathology and AFIP; 2011.
23. Ingles DJ, Pierce Campbell CM, Messina JA, et al. Human papillomavirus virus (HPV) genotype- and age-specific analyses of external genital lesions among men in the HPV Infection in Men (HIM) Study. J Infect Dis. 2015;211:1060-7.
24. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: The HIM study. Eur Urol. 2015;69:166-73.
25. Fernandez-Nestosa MJ, Guimera N, Sanchez DF, et al. Detection of HPV genotypes according to subtypes of penile intraepithelial neoplasia (PeIN). A study of 126 lesions in 43 patients using laser capture microdissection (LCM) PCR [abstr]. Mod Pathol. 2015;28:219-20A.
26. Fernandez-Nestosa MJ, Guimera N, Sanchez DF, et al. Warty penile intraepithelial neoplasia: morphological and viral characterization in 24 cases using laser capture microdissection (LCM)-PCR [abstr]. Mod Pathol. 2016. [In press]
27. Baydar DE, Kulac I, Ozagari A, Guler Tezel G. Occurrence of dysplasia and human papilloma virus typing in penile condylomas. Urology. 2013;81:211.e9-15.
28. Bleeker MC, Hogewoning CJ, Van Den Brule AJ, et al. Penile lesions and human papillomavirus in male sexual partners of women with cervical intraepithelial neoplasia. J Am Acad Dermatol. 2002;47:351-7.
29. Bleeker MC, Snijders PF, Voorhorst FJ, Meijer CJ. Flat penile lesions: the infectious “invisible” link in the transmission of human papillomavirus. Int J Cancer. 2006;119:2505-12.

2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.