Will We Be Able To Care For Cancer Patients In The Future?
The number of cancer patients and cancer survivors continues to increase rapidly amid predictions of a shortfall in physicians to care for them. In addition, newer cancer therapies have become increasingly complex and resource-intensive, compounding the impending workforce shortage. Simultaneously, the growing understanding of the biologic heterogeneity of cancer and the development of pharmacogenomics have opened up the possibility of personalized approaches to cancer diagnosis and treatment. Such personalization has been promulgated as a means of decreasing the cost of drug development, improving the efficacy of treatments, and reducing treatment toxicity. Although there have been notable successes, the fulfillment of these promises has been inconsistent. Providing care for future cancer patients will require the development of innovative delivery models. Moreover, new approaches to clinical research design, to the assessment of therapeutic value, and to the approval of and reimbursement for diagnostics and treatments are needed.
The number of cancer patients and cancer survivors continues to increase rapidly amid predictions of a shortfall in physicians to care for them. In addition, newer cancer therapies have become increasingly complex and resource-intensive, compounding the impending workforce shortage. Simultaneously, the growing understanding of the biologic heterogeneity of cancer and the development of pharmacogenomics have opened up the possibility of personalized approaches to cancer diagnosis and treatment. Such personalization has been promulgated as a means of decreasing the cost of drug development, improving the efficacy of treatments, and reducing treatment toxicity. Although there have been notable successes, the fulfillment of these promises has been inconsistent. Providing care for future cancer patients will require the development of innovative delivery models. Moreover, new approaches to clinical research design, to the assessment of therapeutic value, and to the approval of and reimbursement for diagnostics and treatments are needed.
The future of cancer care is uncertain. Never before have we had so many drugs with which to treat a burgeoning population of cancer patients. Our improved understanding of the biologic heterogeneity of cancer, and the promise-sometimes specious-of pharmacogenomics to enable a personalized approach to diagnosis and treatment, are accompanied by public expectation of these improvements, but also a low tolerance for their soaring costs. With the aging of the population and the success of improved screening and diagnostics, the number of cancer patients is growing; with improved treatments, the population of survivors continues to grow as well. The number of providers to care for both, however, remains relatively stable. What then, is the future of this increasingly personalized, effective, labor-intensive, and costly cancer care for the growing population of cancer patients and survivors?
Increasing Demand for Care
An aging population, improved screening, and more effective therapies have resulted in a steadily increasing number of patients and cancer survivors. The incidence of newly diagnosed cases of cancer, although decreased slightly over the last decade, is now more than 1.5 million new cases per year.[1,2] During this same time period, more precipitously declining mortality rates, resulting from early detection and improved therapies, have left the number of patients dying of cancer at fewer than 600,000 per year. With time, these trends will significantly increase the overall number of cancer patients and survivors actively undergoing treatment or surveillance.
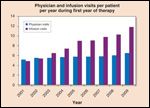
In addition to the increased number of cancer patients, newer cancer therapies have increased the complexity and needs of these patients, resulting in greater utilization of physician, nurse, and other health care resources per patient per unit of time. An academic cancer center in New England tracked these data and determined that in the past 9 years (2001 to 2009), the average number of physician visits per patient per year rose 28% during the first year of treatment (Figure 1; Lawrence N. Shulman, MD, personal communication, June 2010). Moreover, the number of infusion visits per patient during the first year of therapy increased by 147%. In addition to a greater intensity of resource utilization per patient, improved cure rates, prolonged survival, and increased availability of additional therapies for patients not cured, all result in a significant increase in the number of patients requiring short- and long-term care, even without consideration of the number of newly diagnosed patients. The increase in the number of patients with cancer at the same New England academic cancer center is shown in Figure 2A. Although the number of new patients seen each year increased modestly during the period 2001 to 2009, the number of patients receiving ongoing care rose 127%.
These data are even more striking for a disease such as breast cancer, for which long-term survival rates are greater than 80% (Figure 2B). The number of new breast cancer patients increased by 22% over the 9-year period, while the number of patients receiving ongoing care increased by more than 175%. Although such increases may be less pronounced for other diseases, for which survival rates are lower, the same general tenets apply. Additional improvements in treatments and outcomes will only accelerate these trends.
The hope that cancer would be able to be managed with oral agents, thereby diminishing the demand for infusion room visits, remains unfulfilled. Although the number of oral cancer therapies continues to increase, there has been an even more dramatic increase in the number of parenteral medications that require infusion room visits and close monitoring. In the past decade, the number of approved new parenteral agents was more than double that of oral anticancer agents.[3]
Physician Shortage
Studies have predicted that, within the next few years, the number of oncologists will be insufficient to meet the needs of patients with cancer and of cancer survivors.[4,5] The aggregate of the factors detailed above will exacerbate further the discrepancy between the number of patients requiring care and the number of medical oncologists available to provide that care.

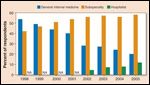
It has been proposed that ongoing follow-up care for cancer survivors should be transitioned to their primary care physicians and/or other providers soon after completion of their treatment, thereby freeing oncologists to concentrate their efforts on caring for patients undergoing active therapy. There are two major obstacles to this approach. First, survivorship is a distinct phase for cancer patients during which they face significant physical and emotional challenges both in the short-term and long-term-and for which coordination of care between specialists and primary care providers is essential, but often missing.[6,7] Multiple Institute of Medicine committees and workshops have identified important gaps in the care of these patients. Perhaps even more daunting for the proposal to transition survivorship care to primary care providers are the data, shown in Figure 3, that demonstrate the decreasing number of trainees entering internal medicine without subspecialty training.[8] Coincident with this, recently passed health care legislation will allow millions of previously uninsured patients to avail themselves of health care coverage. Precisely at a time when the demand for, and demands on, internists are increasing, the number of primary care physicians is declining, thereby increasing further the demands on those who remain.[9,10,11] In addition to caring for the increased demands of an aging population, primary care physicians also must contend with the rising number and complexity of pay-for-performance and health maintenance initiatives, and stay abreast of an ever-evolving medical literature. Like the medical oncologist, the future primary care physician may face the Hobson’s choice of caring for patients with acute medical illnesses or cancer survivors who may be relatively well but at risk for cancer or treatment-related medical problems that require ongoing surveillance. It is likely, and not inappropriate, that patients requiring acute intervention will be given priority.
Cancer Survivorship Care

There is no coordinated strategy that is likely to increase the supply of oncologists or primary care physicians to adequately provide for the looming needs and number of cancer patients and cancer survivors in the coming decade. New, innovative models of care will need to be explored, and several cancer centers are beginning to develop new models of care utilizing “physician extenders,” such as nurse practitioners, physician assistants, and registered nurses. The use of such models may help solve at least the workforce deficiency in oncology. A collaborative practice model, in which physicians partner with such extenders, allows physicians to care for new patients and for patients with complex problems, while the extenders follow patients in treatment and provide survivorship care. In some centers, physician extenders practice independently and are able to bill for their services.
Similar models are being explored in survivorship clinics to address the gaps associated with transition of the cancer survivor’s care from active treatment to follow-up. In this model, the physician extender meets with the patient at the completion of treatment to provide a formal end-of-treatment summary and follow-up care plan for the patient. Follow-up care plans are based on data-driven guidelines developed by oncologists, appropriate subspecialists, and primary care physicians, and are linked to quality-of-care measures similar to those used for other chronic diseases.[12,13,14] Such a plan facilitates the transition from the oncologist to the primary care provider. In addition to summarizing the patient’s treatment, the document serves as a tool for both the patient and the primary care provider to identify relevant issues and provide guidance regarding the appropriate follow-up care specific to the patient’s particular cancer, treatment, and associated medical risks. Further efforts to educate primary care physicians and primary care physician extenders about cancer survivorship issues and to develop cohorts of primary care physicians and extenders interested in survivorship care also would help to address the knowledge and coordination gaps that accompany the transition of care from active treatment to ongoing follow-up.
Improving Cancer Treatments and the Promise of Personalized Medicine
The past decade has witnessed an explosion of knowledge and research into the biologic heterogeneity of cancer that has contributed to a deeper understanding of the differences in clinical presentation, prognosis, response to and tolerance of therapy, and risk for recurrence among persons with the same apparent cancer type. Cancer is no longer defined simply by its organ of origin; instead, it is now subdivided by biologic variables that blur old boundaries and identify numerous new subtypes that may differ dramatically in their presentation, behavior and response to therapy. With this increased understanding has come the development of newer, targeted agents that are designed to take advantage of these biologic differences. Indeed, the era of developing nonspecific, non-targeted chemotherapeutic agents to use against a broad spectrum of tumor types has largely ended; it has been replaced by one characterized by therapies directed against a particular molecular target or pathway with the promise of increased efficacy and decreased toxicity. However, the fulfillment of this promise has been inconsistent. Although there have been successes, most notably the use of trastuzumab (Herceptin) in patients with HER2-positive breast cancer, more often the benefits of these agents have been modest, frequently associated with nontrivial toxicity, and accompanied by very high costs.
The use of bevacizumab (Avastin) in breast cancer is illustrative. In 2008, the FDA approved bevacizumab in combination with paclitaxel for the first-line treatment of metastatic breast cancer based on prolongation of progression-free survival (PFS) compared with paclitaxel alone (median PFS, 11.8 vs 5.9 months; hazard ratio [HR], 0.6; P < .001).[15] Though not included in the original application for FDA approval, results from the AVADO trial, another randomized clinical trial that compared docetaxel (Taxotere) and bevacizumab to docetaxel alone, were also available. This latter study demonstrated a statistically significant improvement in PFS of less than one month with the addition of bevacizumab (PFS, 8.8 vs 8 months; HR, 0.61; P = .0001). Neither trial demonstrated survival benefit. A third trial, the RIBBON-1 study, also demonstrated improved PFS, in this case varying from 2.4 to 3.6 months, depending on the agent used in conjunction with bevacizumab; however, it too showed no survival benefit associated with the use of bevacizumab. At the American Society of Clinical Oncology (ASCO) 2010 conference, a meta-analysis of these studies was presented that demonstrated improved response rates and PFS, but no evidence of a survival benefit from the addition of bevacizumab to chemotherapy for patients with metastatic breast cancer. In the meantime, bevacizumab, the cost of which is ~$90,000 per year, has been approved and used for the treatment of metastatic breast cancer. With approximately 45,000 women diagnosed annually with metastatic breast cancer, the potential cost of this therapy to the health care system is significant.
A similar example is the approval of bevacizumab for the treatment of locally advanced or metastatic non–small-cell lung cancer (NSCLC) based on an overall survival (OS) benefit of 2 months.[16] This story can be repeated with multiple agents. The EGFR inhibitor cetuximab (Erbitux) received accelerated approval in 2004 for the treatment of advanced colorectal cancer based on an improvement in response rate from 10.8% to 22.9% for the combination of cetuximab and irinotecan (Camptosar) compared with cetuximab alone in patients who had received prior irinotecan therapy. Median duration of response was increased by 1.6 months, and the time to disease progression was increased from 1.5 months to 4.1 months.[17]
In 2007, the FDA granted cetuximab regular approval based on a National Cancer Institute of Canada study of best supportive care vs cetuximab monotherapy in patients with heavily pretreated metastatic colorectal cancer. The study demonstrated a survival advantage of 1.5 months in favor of cetuximab therapy.[18] The cost of 2 months of cetuximab therapy (the median progression-free survival in the study) for metastatic colorectal cancer is ~$37,000. A crude, and conservative, estimate utilizing an average patient with a body surface area of 1.5 m2, treated according to the regimen with the dose intensity reported in the published trial, yields a cost per year of life saved by cetuximab of nearly $300,000. The propensity of new agents to command high price tags is becoming well-established. More than 90% of the anticancer agents approved by the FDA over the last several years cost more than $20,000 for a 12-week treatment course.[19]
In addition to allowing treatment to be tailored to a particular patient’s tumor characteristics or selecting the right drug at the right dose to improve the patient’s outcome, one of the promises of personalized medicine is that the improved understanding of the cancer genome will enhance the efficiency of the drug development process. This might be accomplished by using pharmacogenomic information to enrich a study population with potential responders, thereby permitting more limited enrollment and potentially shorter clinical trials.
This potential was fulfilled in the case of the development of trastuzumab in breast cancer, in which only patients with HER2-positive breast cancer were enrolled in a randomized trial to evaluate treatment with this anti-HER2 therapy. This allowed the study to be conducted with a quarter of the number of patients that would normally have been required-and also enabled the study to be completed in one-fifth the time.[20] Trastuzumab was subsequently brought to market in a fraction of the time and for a fraction of the cost that otherwise would have been required. Unfortunately, the synchronized development of a drug and of a corresponding pharmacogenomic test has not been the norm. In the case of cetuximab, it was four and a half years from initial approval until the results of a retrospective study demonstrated that patients with mutated K-ras tumors failed to benefit from cetuximab administration. Once it was understood that this biomarker could identify patients unlikely to benefit from the therapy, the cost-effectiveness of the drug improved dramatically.
Another example in which the development and validation of pharmacogenomics testing have added complexity and cost to the drug development process was that of the epidermal growth factor receptor (EGFR) inhibitor gefitinib (Iressa). In 2003, after gefitinib had demonstrated a response rate of 10% in early clinical trials, the drug was deemed to show sufficient clinical activity to receive accelerated approval for the treatment of NSCLC.[21] Subsequent randomized clinical trials demonstrated no survival benefit, and approval was ultimately withdrawn.[22,23] Eventually, EGFR mutations that better predicted benefit from the EGFR inhibitors were identified.[24] To date, the FDA has not revisited approval of the drug.
So, although pharmacogenomics hold the potential to improve the success, efficacy, and development costs of drugs, such benefits are not certain. Even if the use of pharmacogenomics is successful at reducing the cost of drug development, there is no guarantee that this savings will be passed on to consumers. Pharmaceutical companies endeavor to develop therapies that will extend and improve life, but they are also private enterprises that are charged with creating value for their shareholders.
Impediments to Assessing and Creating Value in Cancer Treatments
The means of assessing the benefits of cancer treatments, and the connection between this assessment and approval by regulatory authorities and adoption by the oncology community are not well defined. In practice, the assessment of a treatment frequently relies on a demonstration of improvement in OS, regardless of how small-although clinical surrogates, often with a tenuous connection to survival, are also used. Unfortunately, these measures may not be sufficient to truly assess the value of cancer treatments.
Assessing the value of cancer treatments

necessitates knowing the clinical benefits and risks for a typical patient population. Clinical cancer research, however, is not oriented in such a way as to yield this information. According to Mullins and colleagues, the incentives for the “clinical research enterprise” favor the performance of trials, typically randomized controlled trials, that produce evidence that will lead to drug approval by the FDA and ultimately to maximal market penetration.[25]
This type of evidence achieves internal validity at the expense of generalizability, and is therefore less relevant to real-world treatment decisions than are data on real-world patient care. The frequent use of approved agents for poorly-evaluated off-label indications and the accelerated approval process compound the lost opportunity to contribute to generalizable knowledge by failing to collect data on patients who are treated for off-label indications or subsequent to drug approval. Given that each newly approved therapy becomes a potential adjunctive line of therapy for patients, the cumulative cost of treatment per patient rises, generally without any contribution to the knowledge of whether this additional therapy has any marginal real-world benefit and without any evaluation of the additional and cumulative costs.
Potential Options for Assessing and Optimizing Value
Assessing the value of cancer treatments will require collecting data about the real-world benefits and costs of cancer treatments. The determination of benefits should include health outcomes as well as quality-of-life measures, and the costs should include both the risks and side effects for patients, as well as the economic costs of therapy. These data can be used to conduct comparative effectiveness research (CER) to evaluate the effectiveness of alternative approaches to the prevention, diagnosis, and treatment of clinical diseases; the results would inform both clinical decisions and policy determinations.[26] Far from conflicting with the tenets of personalized medicine, CER provides more and better information that can be used to develop management strategies for individual patients and that ultimately may facilitate the discovery of the best applications of personalization. In recognition of these benefits, recent health care legislation has directed new funding toward CER. So-called “pragmatic” clinical trials-prospective clinical trials that compare clinically relevant alternative therapies chosen based on common decision-making scenarios in generalizable populations, and that assess clinically relevant outcomes for patients, clinicians, and payers-will also provide real-world data that can help assess the value of different therapies. “Coverage with evidence development” (CED) policies that provide for conditional coverage for promising treatments, but only for patients who are included in a study, would allow for broad access to promising therapies while creating an incentive for participation in trials that will yield answers about the “real-world” clinical benefits or harm of a diagnostic or therapeutic.[25]
Such policies could take the place of accelerated approvals, which provide access but yield no information from the treatment of patients not treated in clinical studies, thereby failing to provide data that could be useful for decisions about drug value. Consideration may also be given to creation of a US entity similar to the National Institute of Clinical Excellence (NICE) in the United Kingdom, an organization charged with evaluating clinical evidence and making policy recommendations regarding approval and reimbursement of therapies. Such an evaluation should include a cost-utility analysis (eg, quality-adjusted life years [QALYs]) with a benchmark (in the United Kingdom it appears to be approximately £30,000 per QALY, although NICE purportedly does not accept or reject treatments or technologies based solely on cost-effectiveness). It has been hypothesized that such a cost-utility benchmark might actually prompt innovation, by directing research endeavors toward the development of interventions that produce greater improvements than a 1- to 2-month survival benefit, or at least price marginal improvements at a level more appropriate to their conferred benefits.
Conclusion
The challenges we face as we attempt to fund and provide cancer care for our future patients are daunting. Nevertheless, we must not lose sight of the fact that we face these challenges, in large part, because of our past success. Our understanding of cancer has increased dramatically in the past two decades. Our ability to affect the course of these complicated diseases has led to improved prognoses and increased survival. The challenge of caring for a growing population of survivors exists because our care has enabled more people to survive. The increased cost of drugs is, at least to some extent, a result of the cost of the research that has enhanced our knowledge of the intricacies of the many different cancers and our ability to exploit these to the patient’s benefit. In short, the challenges we face now stem from turning the challenges we faced previously into opportunities. The good news is that there is no shortage of opportunities confronting us.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. National Cancer Institute. Cancer trends progress report - 2009-2010 update. Available from: http://progressreport.cancer.gov/doc_detail.asp?pid=1&did=2009&chid=93&coid=920&mid=#key.
2. American Cancer Society. Cancer Facts & Figures 2010. Available from: http://www.cancer.org/Research/CancerFacts Figures/CancerFactsFigures/cancer-facts-and-figures-2010.
3. Data derived from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Reports.ReportsMenu
4. Erikson C, Salsberg E, Forte G, et al. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Oncol Practice. 2007;3:79-86.
5. Warren J, Mariotto A, Meekins A, et al. Current and future utilization of services from medical oncologists. J Clin Oncol. 2008;26:3242-7.
6. Hewitt M, Greenfield S, Stovall E: From cancer patient to cancer survivor: Lost in transition. Washington, DC National Academies Press, 2006.
7. Shulman LN, Jacobs LA, Greenfield S, et al. Cancer care and cancer survivorship care in the United States: will we be able to care for these patients in the future? J Oncol Practice. 2009;5:119-23.
8. Bodenheimer T: Primary care: will it survive? N Engl J Med. 2006;355:861-4.
9. Salsberg E, Grover A. Physician workforce shortages: implication and issues for academic health centers and policymakers. Acad Med. 2006;81:782-7.
10. Goodman D, Fisher E. Physician workforce crisis? Wrong diagnosis, wrong prescription. N Engl J Med. 2008;358:1658-61.
11. Starfield B, Fryer G. The primary care physician workforce: ethical and policy implications. Annals of Family Med. 2007;5:486-91.
12. Earle CC, Nevill BA. Underuse of necessary care among cancer survivors. Cancer. 2004;101:1712-19.
13. Nissen M, Beran M, Lee M, et al. Views of primary care providers on follow-up care and cancer patients. Family Medicine. 2006;39:477-482, 2006.
14. Earle CC, Burstein HJ, Winer EP, et al. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2010;21:1447-51.
15. Gray R, Bhattacharya S, Bowden C, et al. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27:4966-72.
16. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2010;355:2542-50.
17. Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-45.
18. Jonker DJ, Callaghan C, Karapetis C, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040-8.
19. Cohen H. Drug topics Red Book™ 2008. 112th ed. Montvale, NJ: Thomson Healthcare/Thomson PDR; 2008.
20. Press M, Seelig S, Lessons learned from the development of a diagnostic to predict response to Herceptin: targeted medicine-from concept to clinic. Thomson Financial Street Events, Conference Report on Targeted Medicine. 2004;10-11.
21. Kris M, Natale RB, Herbst R, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non–small cell lung cancer. JAMA. 2003;290:2149-58.
22. Giaccone G, Herbst R, Manegold, C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small cell lung cancer: a phase III trial-INTACT 1. J Clin Oncol. 2004;22:777-84.
23. Herbst R, Giaccone G, Schiller J, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small cell lung cancer: a phase III trial-INTACT 2. J Clin Oncol. 2004;22:785-794.
24. Mok T, Wu Y-L, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-57.
25. Mullins CD, Montgomery R, Tunis S. Uncertainty in assessing value of oncology treatments. Oncologist. 2010;15(Suppl 1):58-64.
26. Garber AM, Tunis S. Does comparative-effectiveness research threaten personalized medicine? N Engl J Med. 2009;360:1925-7.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.