13 Real-world Treatment Patterns and Tumor Response of Palbociclib Plus an Aromatase Inhibitor for Metastatic Breast Cancer: Flatiron Database Analysis
Debra Patt, MD, PhD1; Xianchen Liu, MD, PhD2; Benjamin Li, PhD3; Lynn McRoy, MD2; Rachel M Layman, MD3; Adam Brufsky MD, PhD4
1Texas Oncology-Austin Central, Austin, TX
2Pfizer, New York, NY
3The University of Texas MD Anderson Cancer Center, Houston, TX
4University of Pittsburgh School of Medicine, Pittsburgh, PA
Background
The use of a CDK4/6 inhibitor in combination with an aromatase inhibitor (AI) as initial endocrine therapy has become the standard of care for patients with hormone receptor–positive, HER2-negative (HR+/HER2–) advanced or metastatic breast cancer (mBC). This study described treatment patterns and real-world tumor response (rwTR) to first-line palbociclib (Ibrance; PA) plus AI therapy for HR+/HER2– mBC in a routine clinical setting in the United States.
Methods
This was a retrospective analysis of Flatiron Health’s nationwide longitudinal electronic health records from over 280 cancer clinics, representing more than 2.2 million actively treated patients with cancer in the United States. There were 813 women with HR+/HER2– mBC, initiated with PA plus AI as first-line therapy between February 2015 and September 2018 with at least 3 months of potential follow-up. Patients were followed from the start of PA plus AI therapy to December 2018, death, or last visit, whichever came first. The rwTR that occurred at least 30 days after the date of therapy initiation was assessed based on the treating clinician’s assessment of radiologic evidence for change in burden of disease over the course of treatment.
Results
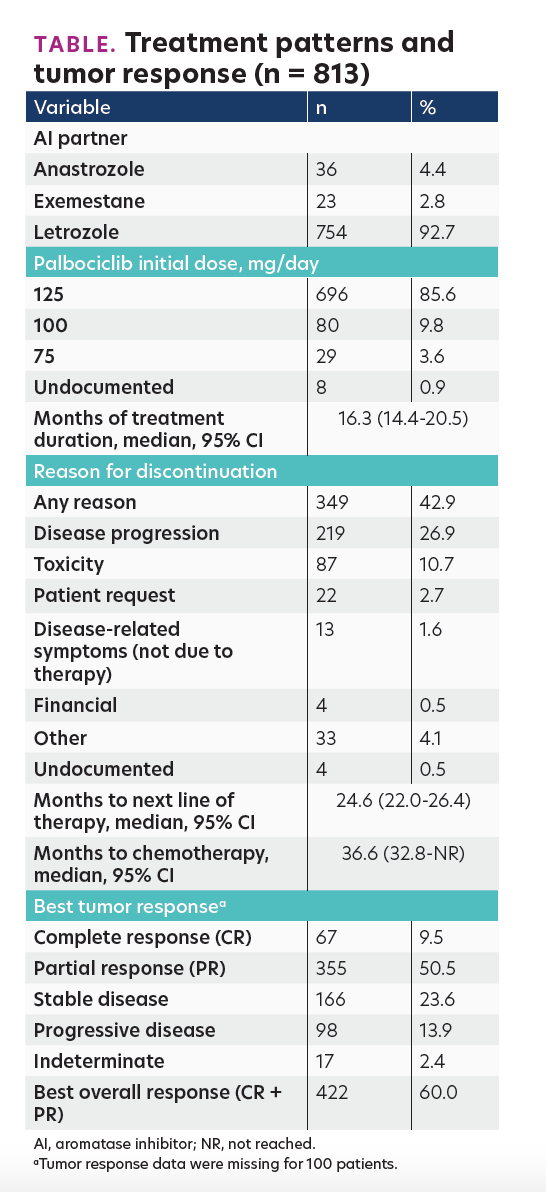
Among 813 eligible patients, 68.3% were white, the median age was 65.0 years, 42.9% had visceral disease (lung and/or liver), 85.6% started PA at 125 mg/day, and the median duration of follow-up was 19.4 months. Median treatment duration and time to chemotherapy were 16.3 months (95% CI, 14.4-20.5 months) and 36.6 months (95% CI, 32.8-not reached), respectively. In total, 42.9% patients discontinued PA plus AI for any reason and 10.7% for toxicity. The rwTR rate was 60%. The Table presents the results in detail.
TABLE. Treatment patterns and tumor response (n = 813)
Conclusions
The real-world findings in heterogeneous patients with mBC in routine clinical practice in the United States support first-line PA plus AI as the standard care for HR+/HER2– mBC.
