19 Efficacy, Safety, and Quality of Life With Ribociclib + Endocrine Therapy in Elderly Patients With HR+/HER2– Advanced Breast Cancer Across the MONALEESA-2, -3, and -7 Trials
Background
Ribociclib plus endocrine therapy (ET) showed significant progression-free survival (PFS) and overall survival (OS) benefits in the MONALEESA (ML) trials in patients with hormone receptor–positive/HER2-negative (HER2+/HER2–) advanced breast cancer (ABC). Here, we report efficacy, safety, and quality of life in elderly patients in the ML trials.
Methods
Data were pooled from ML-2, -3, and -7 for patients treated with ribociclib plus ET or placebo plus ET. PFS, OS, time to first chemotherapy (TTC), time to definitive deterioration (TTD) in pain and fatigue scores by ≥20 points were analyzed in patients <65 years, 65-<75 years, and ≥75 years using Kaplan-Meier (KM) methods.
Results
Efficacy Outcomes Between Age Groups
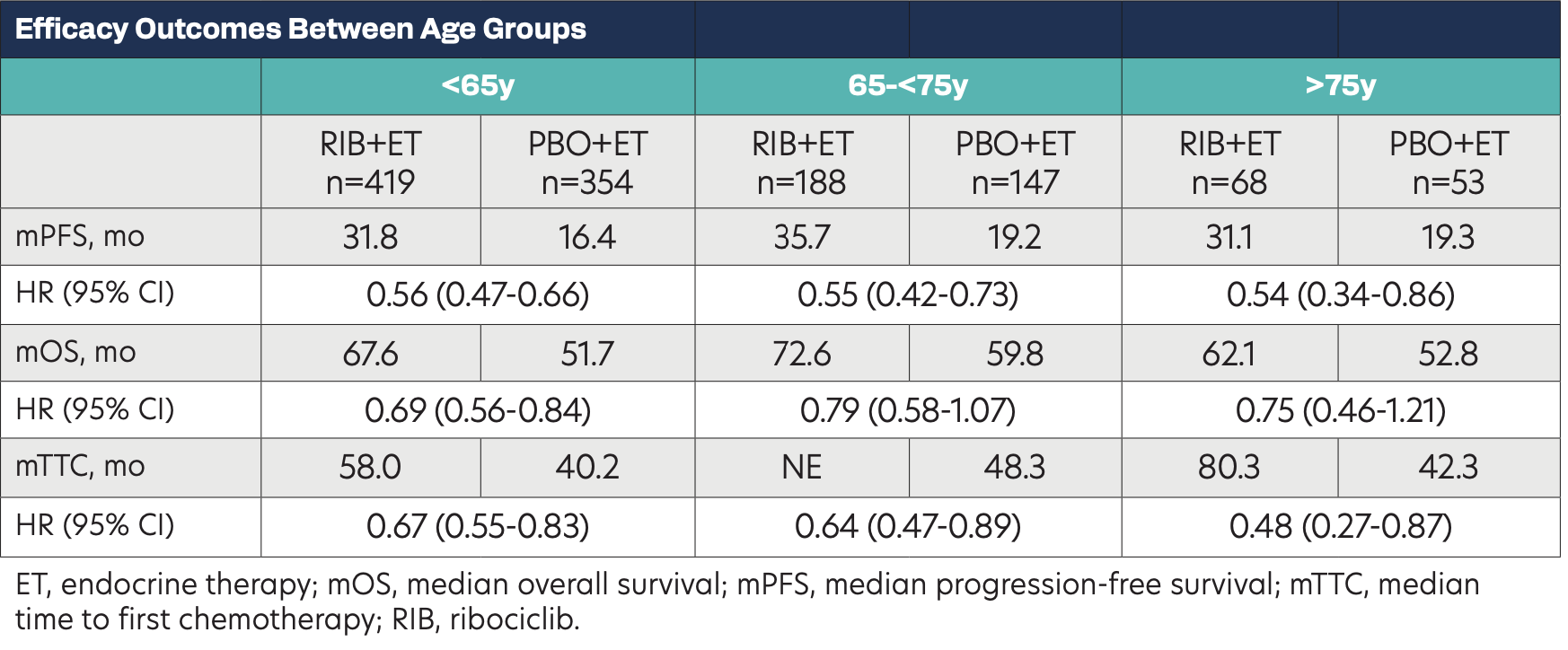
Of the 1229 patients, 63% were <65 years, 27% were 65 years to 75 years, and 10% were ≥75 years. There were minor differences in baseline characteristics between age groups: slightly higher percentage of patients in the ≥75 years group with a ECOG status of 1 and slightly higher percentage of de novo disease in the <65 years group. Regardless of age, a PFS and OS benefit was seen with ribociclib plus ET. Ribociclib plus ET also delayed the median TTC in all age groups. Rates of adverse events (AEs) and most AEs of special interest were generally consistent across all groups. AEs were manageable using dose modifications. In patients who discontinued treatment due to AEs, the percentage of patients without prior dose reduction was 34% in patients <65 years, 41% in patients 65 years to 75 years, and 50% in patients ≥75 years. A TTD benefit with RIB+ET was observed for patients across age groups for pain and fatigue scores.
Conclusions
This pooled analysis of ML-2, -3, and -7 demonstrated consistent efficacy benefits with ribociclib plus ET in all age groups. No new safety signals were identified in elderly patients. A high percentage of elderly patients without dose reduction before discontinuing ribociclib plus ET due to
AEs represents an opportunity to optimize AE management
with dose modifications. This analysis demonstrated that ribociclib plus ET is an effective and well-tolerated treatment for patients of all age groups with HR+/HER2− ABC, including elderly patients.
