3 Things You Should Know About Evolving Strategies in SCLC: Limited-Stage Advances, Frontline Innovation, and Postplatinum Progress
Explore innovative strategies and emerging therapies transforming small cell lung cancer treatment, enhancing patient outcomes and survival rates.
The experts.

LEARNING OBJECTIVES
Upon successful completion of this activity, you should be better prepared to:
• Evaluate current and emerging clinical trial data on novel therapeutic strategies for the management of small cell lung cancer (SCLC)
• Develop proactive approaches to anticipate, mitigate, and manage treatment-related adverse events in patients receiving SCLC therapy
• Integrate evidence from clinical trials into real-world practice to optimize the use of SCLC treatments across different lines of care
RELEASE DATE: July 1, 2025
EXPIRATION DATE: July 1, 2026
Accreditation/Credit Designation
Physicians’ Education Resource®, LLC, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physicians’ Education Resource®, LLC, designates this enduring material for a maximum of 0.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Acknowledgment of commercial support
This activity is supported by an educational grant from AstraZeneca Pharmaceuticals.
Off-label Disclosure and Disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a health care professional relative to diagnostic, treatment, or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members, and do not reflect those of PER® or any company that provided commercial support for this activity.
INSTRUCTIONS FOR PARTICIPATION and HOW TO RECEIVE CREDIT
1. Read this activity in its entirety.
2. Go to https://www.gotoper.com/iaslc25hottopics-sclc-postref to access and complete the posttest.
3. Answer the evaluation questions.
4. Request credit using the drop-down menu.
YOU MAY IMMEDIATELY DOWNLOAD YOUR CERTIFICATE.
Novel therapies and regimens are changing the standard of care treatment in the first line and beyond for patients with both limited-stage and extensive-stage small cell lung cancer (LS-SCLC and ES-SCLC). Here are 3 things you should know about evolving strategies in SCLC.
1. Immunotherapy consolidation is redefining the standard for LS-SCLC.
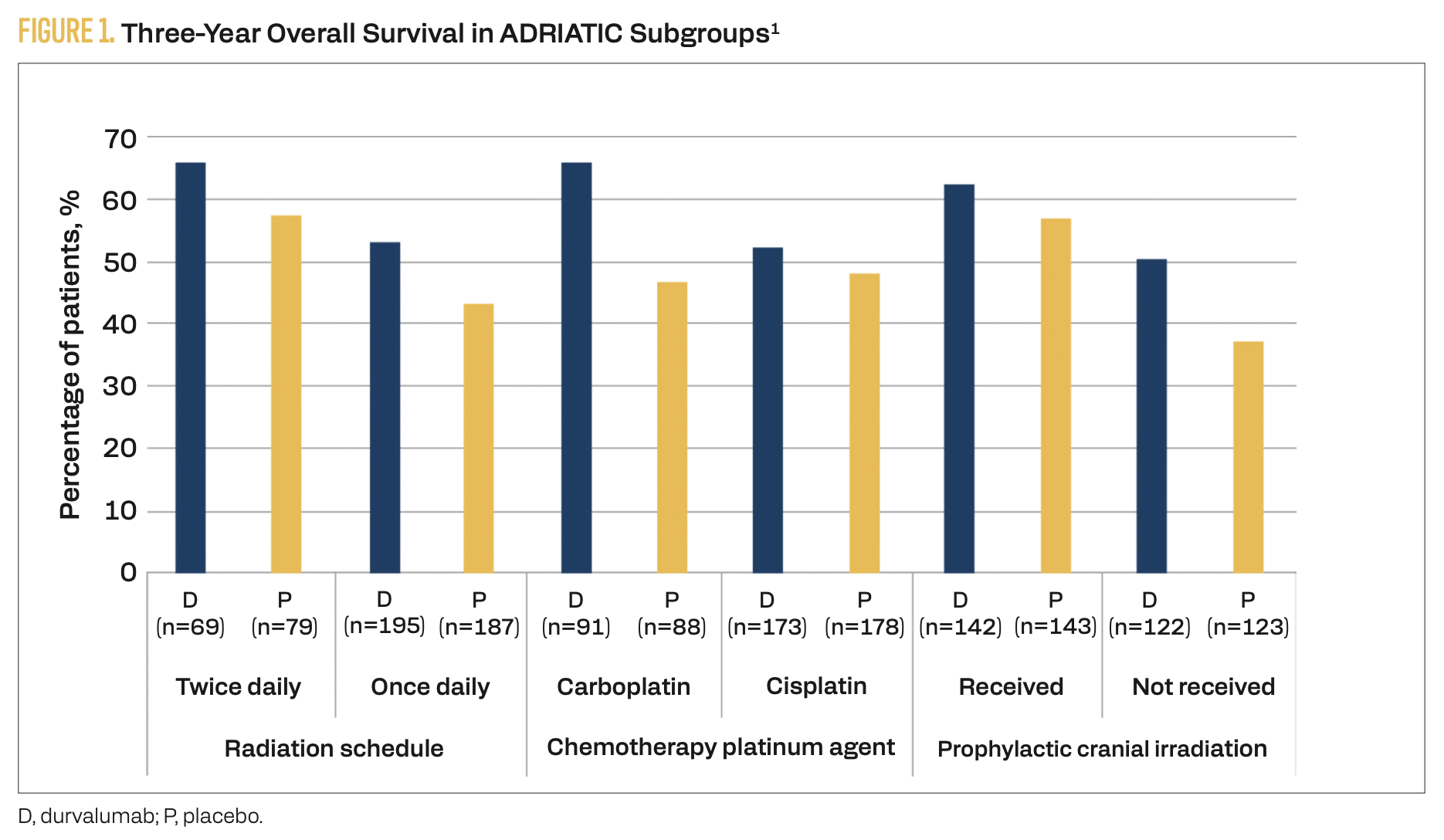
Results from the phase 3, randomized, placebo-controlled ADRIATIC trial (NCT03703297) have established a new treatment option for patients with stage I to III LS-SCLC who have not progressed after concurrent chemoradiotherapy.1 Patients were randomly assigned to receive consolidation therapy with durvalumab (n = 264), durvalumab plus tremelimumab (n = 200), or placebo (n = 266). The median overall survival (OS) with durvalumab was 55.9 months (95% CI, 37.3-not reached) vs 33.4 months (95% CI, 25.5-39.9) with placebo (HR, 0.73; 98.321% CI, 0.54-0.98; P = .01). Subgroup 3-year OS data are summarized in Figure 1.1 The median progression-free survival (PFS) with durvalumab was 16.6 months (95% CI, 10.2-28.2) vs 9.2 months (95% CI, 7.4-12.9) with placebo (HR, 0.76; 97.195% CI, 0.59-0.98; P = .02). Grade 3 or 4 adverse events (AEs) were reported in 24.4% of patients receiving durvalumab consolidation vs 24.2% of patients receiving placebo. Treatment- related AEs led to death in 0.8% of patients receiving durvalumab vs 0% of patients in the placebo arm. Grade 3 or 4 immune-mediated AEs occurred in 53% of patients in the durvalumab arm vs 1.5% of patients receiving placebo. Radiation pneumonitis was the most common AE in the ADRIATIC trial, occurring in 38.2% of patients in the durvalumab arm vs 30.2% of patients in the placebo arm. Most cases were grade 1 or 2.
FIGURE 1. Three-Year Overall Survival in ADRIATIC Subgroups1
2. Frontline chemoimmunotherapy has become standard in ES-SCLC, but challenges remain.
For patients with ES-SCLC, chemoimmunotherapy is now the standard of care, supported by results from pivotal phase 3 trials. The CASPIAN trial (NCT03043872) demonstrated an OS benefit with durvalumab added to chemotherapy vs chemotherapy alone (HR, 0.71; 95% CI, 0.60-0.86; nominal P = .0003), while the IMpower133 trial (NCT02763579) demonstrated an OS benefit with atezolizumab added to chemotherapy vs placebo plus chemotherapy (HR, 0.76; 95% CI, 0.60-0.95; descriptive P = .0154).2,3
The phase 3b MAURIS trial (NCT04028050) evaluated real-world safety and outcomes of atezolizumab plus carboplatin/etoposide in 154 patients with ES-SCLC, including patients with an ECOG performance status of 2 (4%) and/or untreated brain metastases at baseline (8%).4 Serious AEs were reported in 29.9% of patients, and immune-related AEs occurred in 14.9% of patients. The median OS was 10.7 months, and the median PFS was
5.5 months. Tumor responses were seen in 71.6% of patients.
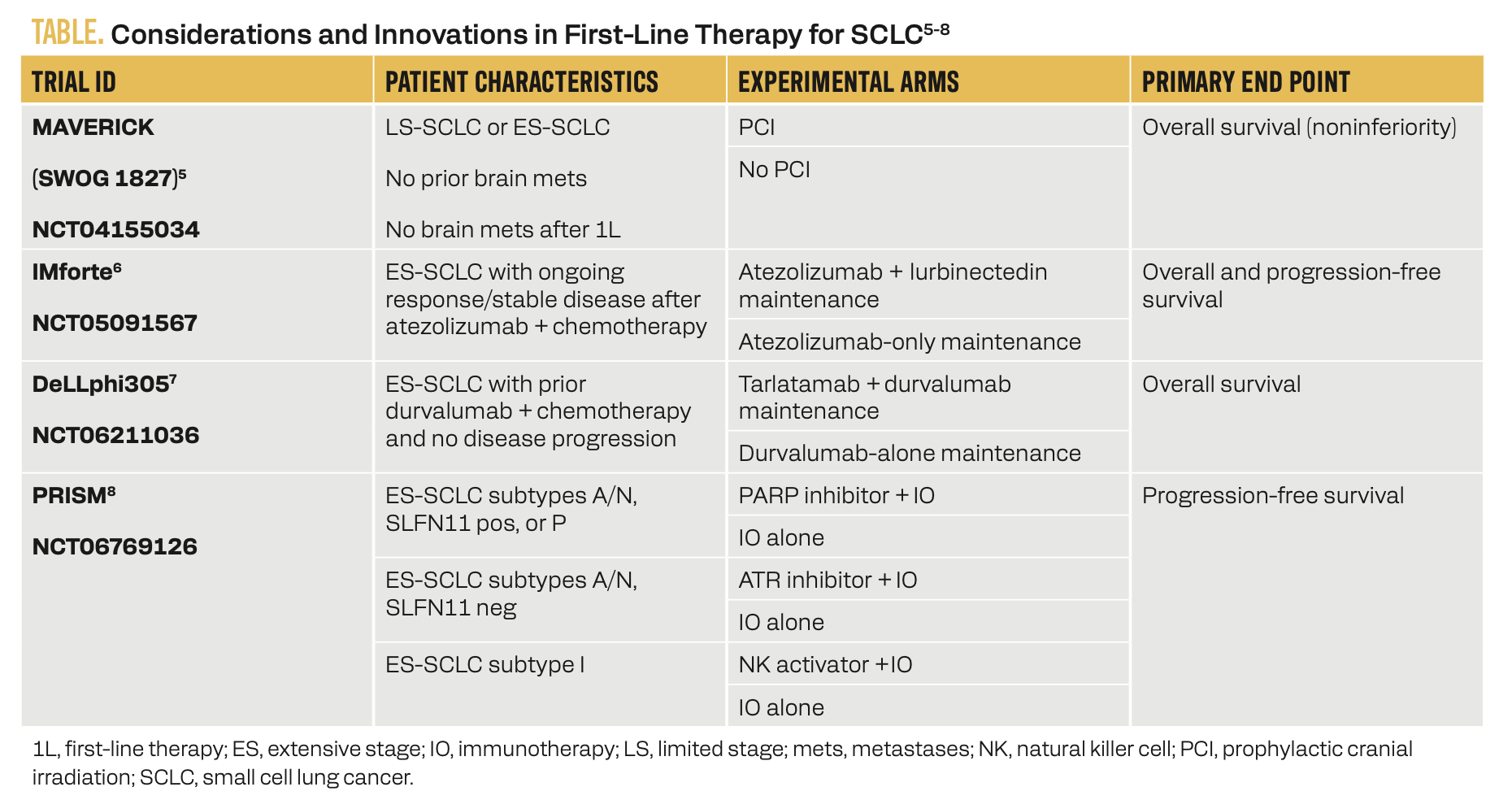
Ongoing trials are addressing remaining gaps in care, including the role of prophylactic cranial irradiation, maintenance strategies, and precision-based first-line approaches (Table).5-8
TABLE. Considerations and Innovations in First-Line Therapy for SCLC5-8
3. Postplatinum options are expanding with targeted therapies and T-cell engagers.
Therapeutic options are increasing for patients with relapsed or refractory SCLC following first-line chemotherapy (Figure 2).9
FIGURE 2. NCCN Guidelines Options for SCLC Subsequent Systemic Therapy9
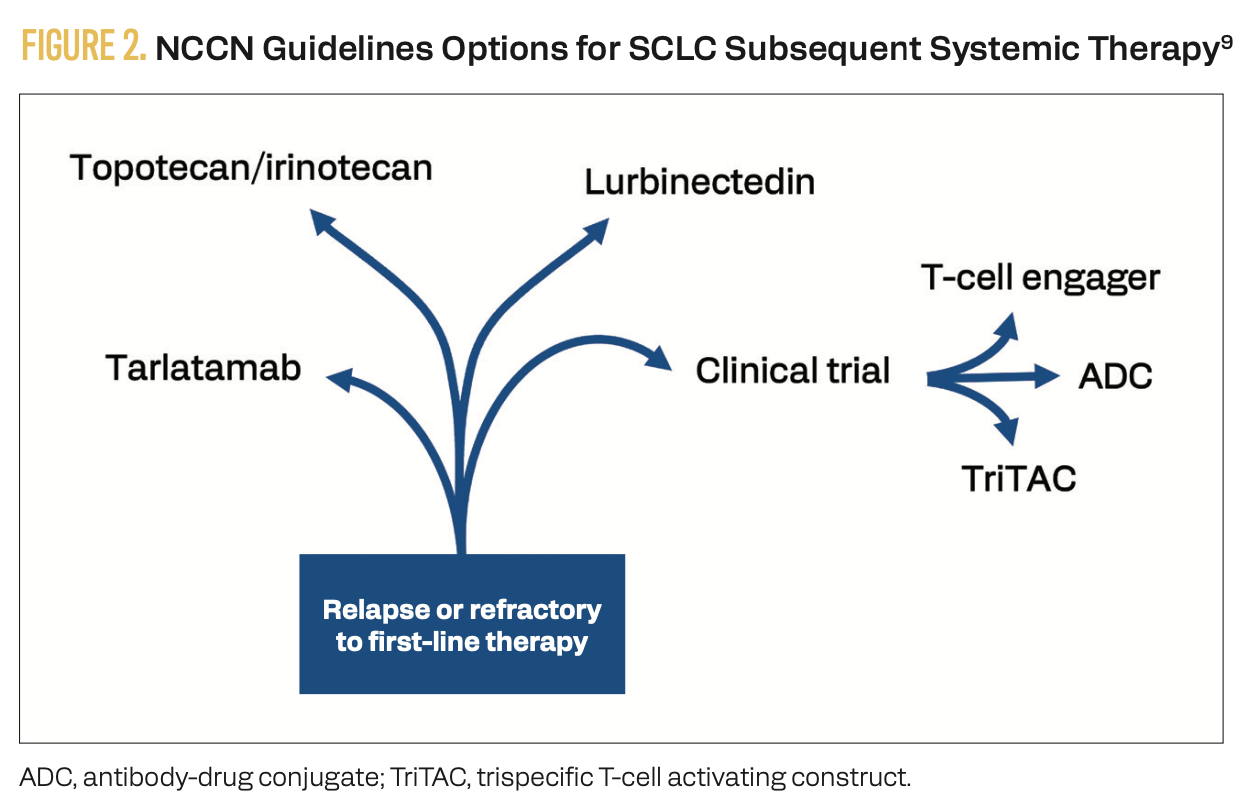
Lurbinectedin remains an important second-line option per NCCN guidelines.9 The phase 1/2 LUPER trial (NCT04358237) studied the safety and efficacy of combination lurbinectedin and pembrolizumab in 12 patients with SCLC who progressed on or after platinum-based chemotherapy.10 One patient experienced complete response, while 3 achieved partial response and another 3 achieved stable disease, for a disease control rate (DCR) of 61.5%. Two patients (15.4%) discontinued pembrolizumab due to immune- related AEs. Dose-limiting toxicities consisted of 1 case of grade 3 asthenia and 1 case of grade 4 neutropenia lasting more than 3 days.
Tarlatamab is a DLL3xCD3 T-cell engager that has been approved for use as a second-line therapy following platinum-based chemotherapy due to the demonstrated sustained clinical benefit in patients with previously treated SCLC in the phase 2 DeLLphi-301 trial (NCT05060016).11 In the 10-mg dose group, the ORR was 40.4% with a median duration of response of 9.7 months (95% CI, 6.9-not estimable [NE]).12 The median PFS was 4.3 months (95% CI, 3.0-5.6), and the median OS was 15.2 months (95% CI, 10.8-NE).
Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) are AEs of particular concern with tarlatamab. In the DeLLphi-301 trial, CRS typically occurred after the first or second dose in cycle 1.12 Overall, 32% of patients experienced grade 1 CRS, while 20% experienced grade 2 CRS and 1% experienced CRS of grade 3. ICANS typically occurred within the first 6 months of treatment. Overall, 10% of patients experienced grade 1 ICANS, and 5% experienced grade 2 events.
Obrixtamig (BI 764532), another DLL3xCD3 bispecific T-cell engager, was studied in a first-in-human phase 1 trial (NCT04429087).13 Among 24 patients with SCLC, the ORR was 33%. Six dose-limiting toxicities were reported, consisting of grade 3 confusion, grades 3 and 4 CRS, grade 3 nervous system disorder, and grade 2 infusion-related reaction. Grade 1 or 2 CRS occurred in 58% of patients and was managed with supportive care, corticosteroids, and/or anti–IL-6R antibodies.
HPN328, a trispecific T-cell activating construct (TriTAC) targeting DLL3, CD3, and albumin, showed promising activity in a phase 1/2a trial (NCT04471727).14 Among 9 evaluable patients with DLL3-positive SCLC, 3 experienced greater than 30% tumor shrinkage. CRS (grade 1 or 2) occurred in 31%, mostly within
24 hours of early doses. Grade 1 or 2 CRS occurred in 31% of patients within 24 hours of the first or second dose.
Additional investigational therapies include antibody-drug conjugates (ADCs) such as ifinatamab deruxtecan (I-DXd), which demonstrated a confirmed ORR of 54.8% (95% CI, 38.7%-70.2%) and DCR of 90.5% (95% CI, 77.4%-97.3%) in 42 patients with ES-SCLC who received the 12-mg/kg dose in the phase 2 IDeate-Lung01 study (NCT05280470).15
ABBV-706 is a SEZ6-targeting ADC that demonstrated an ORR of 40% in 22 patients with SCLC in a first-in-human phase 1 trial (NCT05599984).16 Two patients in the trial experienced a dose-limiting toxicity: 1 case of grade 4 leukopenia and neutropenia lasting more than 7 days and 1 case of grade 4 thrombocytopenia.
Treatment selection after relapse should consider prior therapy, comorbidities, and functional status. The integration of novel targeted agents and T-cell engagers is poised to reshape treatment sequencing and personalization in SCLC.
Key References
1. Cheng Y, Spigel DR, Cho BC, et al. Durvalumab after chemoradiotherapy in limited-stage small-cell lung cancer. N Engl J Med. 2024;391(14):1313-1327. doi:10.1056/NEJMoa2404873
4. Bria E, Morgillo F, Garassino MC, et al. Atezolizumab plus carboplatin and etoposide in patients with untreated extensive-stage small-cell lung cancer: interim results of the MAURIS phase IIIb trial. Oncologist. 2024;29(5):e690-e698. doi:10.1093/oncolo/oyad342
12. Sands J, Cho BC, Ahn MJ, et al. OA10.03 Tarlatamab sustained clinical benefit and safety in previously treated SCLC: DeLLphi-301 phase 2 extended follow-up. J Thorac Oncol. 2024;19(10):S30-S31. doi:10.1016/j.jtho.2024.09.057
For FULL References List, visit https://www.gotoper.com/iaslc25hottopics-sclc-postref
CME Posttest Questions
1 Based on the phase 3 ADRIATIC trial, which of the following statements best reflects the impact of durvalumab consolidation after chemoradiotherapy in patients with LS-SCLC?
A. Durvalumab consolidation showed no statistically significant improvement in overall survival.
B. Durvalumab consolidation led to a 27% reduction in risk of death compared with placebo.
C. The benefit of durvalumab was limited to patients who received twice-daily radiation only.
D. Use of durvalumab was associated with increased grade 4 esophageal toxicity.
2 Which of the following best describes the mechanism of action of obrixtamig in the treatment of SCLC?
A. Selective inhibition of topoisomerase I linked to a membrane-permeable antibody
B. Antibody-drug conjugate targeting SEZ6-positive tumor cells
C. Bispecific T-cell engager that targets DLL3 on tumor cells and CD3 on T cells
D. B7-H3–directed antibody-drug conjugate delivering a cytotoxic payload
3 Which of the following adverse events was most frequently observed in patients receiving durvalumab consolidation therapy in the ADRIATIC trial?
A. Decreased appetite
B. Fatigue
C. Hypothyroidism
D. Radiation pneumonitis
4 A 72-year-old woman with ES-SCLC (T2N3M1a) initially responded to carboplatin, etoposide, and atezolizumab, followed by maintenance atezolizumab. She now presents with disease progression 2 months after completing initial therapy and has an ECOG performance status of 0. Based on recent trial data, which of the following has demonstrated the highest likelihood of longer overall survival?
A. Topotecan
B. Tarlatamab
C. Lurbinectedin
D. Platinum rechallenge
Claim Your CME Credit at https://www.gotoper.com/ iaslc25hottopics-sclc-postref
To learn more about this topic, including clinical case discussions to apply current guidelines and recent data, go to https://www.gotoper.com/ iaslc25hottopics-sclc-activity
CME Provider Contact information
Physicians’ Education Resource®, LLC
2 Commerce Drive, Suite 110, Cranbury, NJ 08512
Toll-Free: 888-949-0045
Local: 609-378-3701
Fax: 609-257-0705
info@gotoper.com
