3 Things You Should Know About Targeting NRG1 and Rare Drivers in Pancreatic Cancer
Explore the latest advancements in pancreatic cancer treatment, focusing on genetic mutations, targeted therapies, and emerging clinical strategies.
The panel

RELEASE DATE: June 1, 2025
EXPIRATION DATE: June 1, 2026
LEARNING OBJECTIVES
Upon successful completion of this activity, you should be better prepared to:
• Evaluate the role of mutations in NRG1 and other genetic alterations in diagnosis and management.
• Discuss current guidelines and treatment recommendations for the management of patients with advanced pancreatic cancer.
• Analyze clinical trial data to inform the selection of emerging therapeutic agents for advanced pancreatic cancer.
• Apply strategies to optimize molecular testing algorithms using diverse testing modalities in advanced pancreatic cancer.
Accreditation/Credit Designation
Physicians’ Education Resource®, LLC, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physicians’ Education Resource®, LLC, designates this enduring material for a maximum of 0.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Acknowledgment of commercial support
This activity is supported by an educational grant from Partner Therapeutics, Inc.
Off-label disclosure/disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a health care professional relative to diagnostic, treatment, or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members and do not reflect those of PER® or any company that provided commercial support for this activity.
Instructions for participation/how to receive credit
1. Read this activity in its entirety.
2. Go to https://www.gotoper.com/annual-oncology-meeting-25-nrg1-postref to access and complete the posttest.
3. Answer the evaluation questions.
4. Request credit using the drop-down menu.
YOU MAY IMMEDIATELY DOWNLOAD YOUR CERTIFICATE.
To date, 32 genes have been identified as frequently mutated in pancreatic ductal adenocarcinoma (PDAC).1 However, less than 10% of patients are eligible for an FDA-approved targeted therapy, highlighting the need for the development of novel therapeutics.2 Here are 3 things you should know about molecular testing and personalized strategies in pancreatic cancer.
1 RNA testing is critical to identify actionable gene fusions in PDAC.
NCCN guidelines recommend tumor molecular profiling in cases of metastatic PDAC.3 RNA-based next-generation sequencing (NGS) is preferred to DNA-based NGS for detecting fusions in genes like ALK, NRG1, NTRK, ROS1, FGFR2, and RET. These fusions are enriched in the 5% to 10% of patients with KRAS wild-type PDAC, a molecular profile more often found in patients younger than 50 years.4 NGS using tumor tissue is preferred to blood-based assays, according to NCCN guidelines.3 Liquid biopsy can be performed concurrently with tissue testing or used when adequate tissue is unavailable.
RNA-based NGS can detect structural variants of gene fusions, which may inform the potential efficacy of targeted therapies.5 Since RNA sequencing encompasses only exons after splicing, this technique can overcome the technical challenges of excessive sequencing or misaligned reads when DNA-based NGS is used on genes with long or repetitive introns (Figure 1).6 In a heterogeneous tumor in which the gene fusion is present only in some cells, RNA-based NGS can detect the alteration if it is highly expressed.
FIGURE 1.RNA-based NGS May Overcome Limitations of DNA-Based Testing6
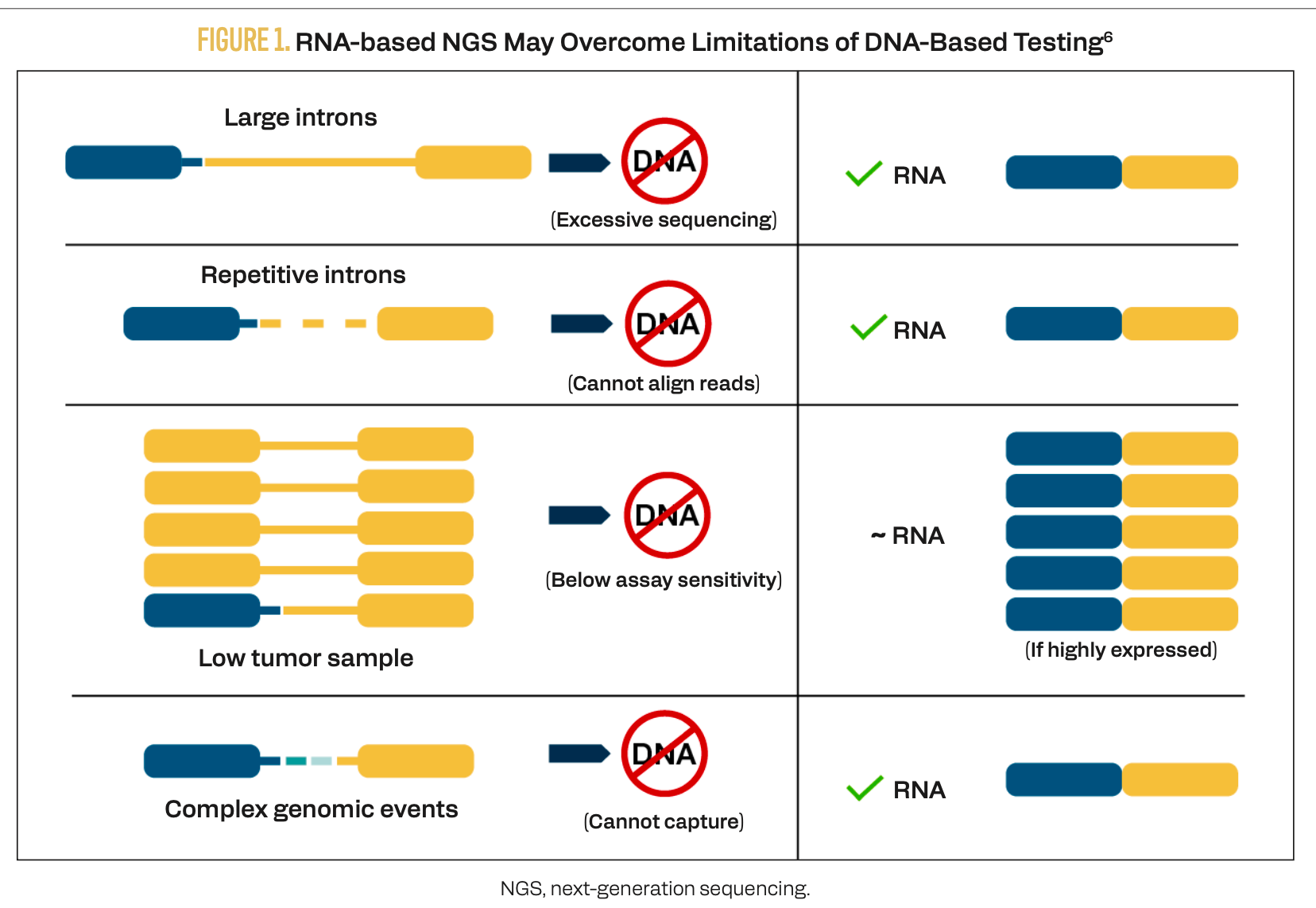
2 The first NRG1-targeted therapy is approved for advanced or metastatic PDAC.
NRG1 fusions are found in approximately 1% of solid tumors, most commonly in patients with mucinous adenocarcinoma of the lung and KRAS wild-type PDAC.7 These altered proteins consist of the EGF-like domain of NRG1 attached to the transmembrane domain of various fusion partners. This construct enables constitutive binding and activation of the HER3 receptor, activating RAS and the MAPK and PI3K signaling pathways.
Zenocutuzumab is a HER2×HER3 bispecific antibody that inhibits NRG1 binding.8 In the phase 1/2 eNRGy trial (NCT02912949), zenocutuzumab demonstrated an overall response rate (ORR) of 30% (95% CI, 23%-37%) in 158 patients of all tumor types with NRG1 fusions.9 The response rate in 33 patients with PDAC was 42.4% (95% CI, 25.5%-60.8%), including 1 complete response and 13 partial responses (Figure 2).10 The median progression-free survival in the overall population was 6.8 months (95% CI, 5.5-9.1 months).9 The most common treatment-related adverse events (TRAEs) were diarrhea (18%), fatigue (12%), and nausea (11%). Infusion-related reactions occurred in 14% of patients. Based on results from the eNRGy study, zenocutuzumab received accelerated approval from the FDA as a second-line systemic therapy to treat advanced, unresectable, or metastatic PDAC harboring an NRG1 gene fusion.11
FIGURE 2. Response to an NRG1 Inhibitor in Pancreatic Adenocarcinoma10
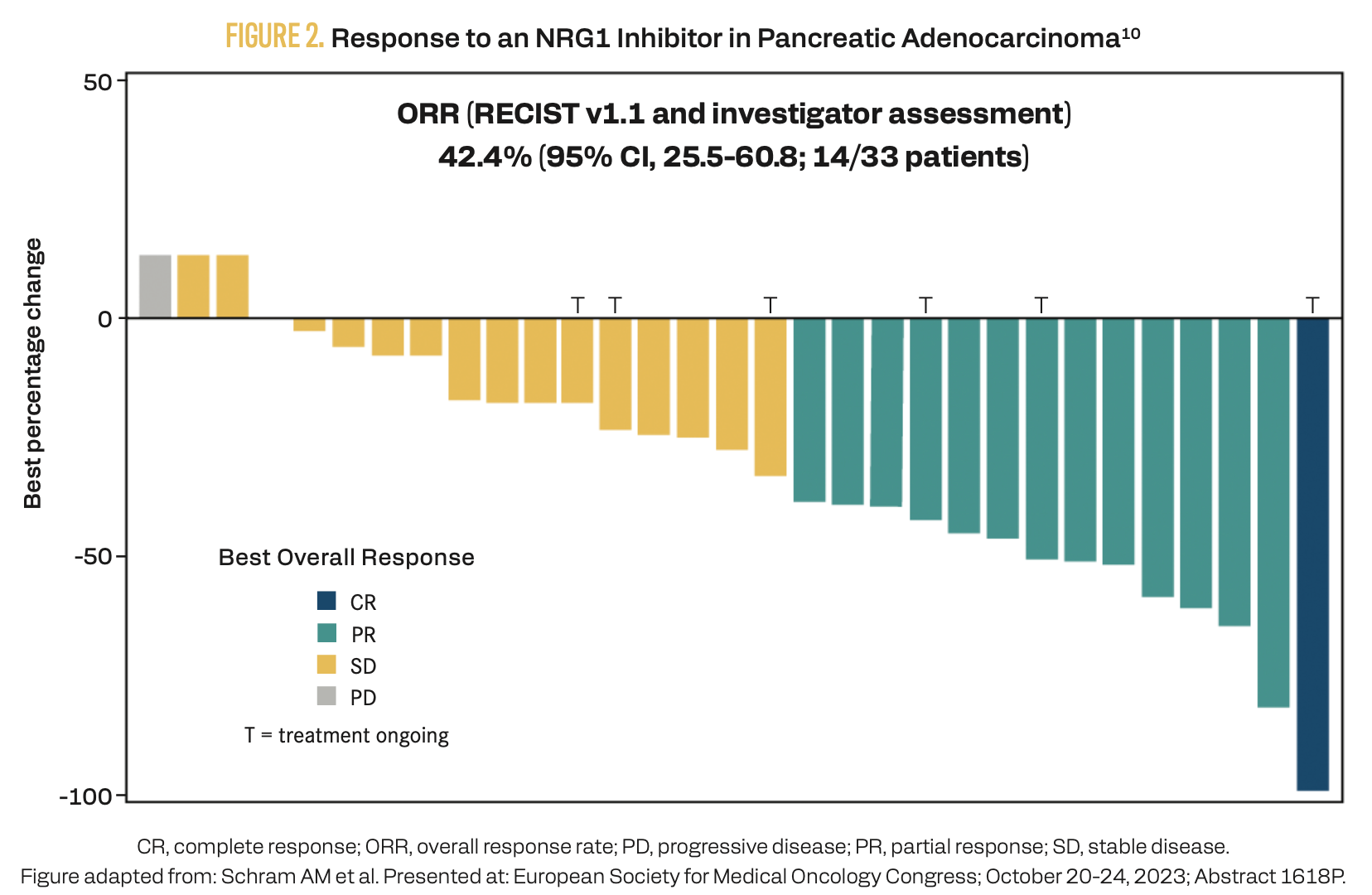
3 Ongoing investigations are evaluating numerous other emerging targets of interest.
In addition to NRG1 fusions, 38.5% of KRAS wild-type PDAC tumors harbor other genetic alterations, including FGFR2 or FGFR3 fusions, ERBB2 (HER2) amplification, BRAF mutations, and RET fusions.4 Erdafitinib demonstrated an ORR of 30% (95% CI, 24%-36%) in patients of different tumor types harboring FGFR alterations in the single-arm, phase 2 RAGNAR trial (NCT04083976).12 The ORR was 56% in patients with PDAC.
Combination dabrafenib and trametinib produced an ORR of 38% (95% CI, 22.9%-54.9%) in patients with solid tumors, lymphomas, or multiple myeloma whose tumors harbored a BRAF V600 mutation in the single-arm NCI-MATCH trial subprotocol H (NCT02465060).13 Of the 27 evaluable patients, the 1 patient with PDAC achieved stable disease. Combined use of dabrafenib and trametinib received accelerated approval to treat patients with unresectable or metastatic solid tumors, including PDAC, that harbor BRAF V600E mutations and who have progressed following prior treatment.14
Selpercatinib yielded an ORR of 43.9% (95% CI, 28.5%-60.3%) in patients with RET fusion-positive non-lung and non-thyroid solid tumors in the phase 1/2 LIBRETTO-001 basket trial (NCT03157128).15 The ORR was 44% in patients with PDAC. Selpercatinib received accelerated approval to treat adults with locally advanced or metastatic solid tumors, including PDAC, that harbor a RET gene fusion and who have progressed following prior treatment.16
The HER2-targeting antibody-drug conjugate (ADC) fam-trastuzumab deruxtecan-nxki (T-DXd) has not provided as much benefit in patients with PDAC. In the phase 2 DESTINY-PanTumor02 trial (NCT04482309) in patients with solid tumors overexpressing HER2 (immunohistochemistry [IHC], 3+ or 2+), the ORR was 37.1% (95% CI, 31.3%-43.2%) across all cohorts, but only 4.0% (95% CI, 0.1%-20.4%) in those with PDAC.17 Further investigation would be required to determine why this disease type is particularly resistant to T-DXd, but this agent is still approved for HER2 IHC 3+ PDAC under the tissue agnostic approval.
The TP53 Y220C mutation is the target of the first-in-class p53 reactivator PC14586.18 In the phase 1 portion of the phase 1/2 PYNNACLE trial (NCT04585750) at the highest dose of PC14586, the ORR was 46.2%. In 6 patients with PDAC, 4 achieved stable disease, and 1 achieved an unconfirmed partial response.
Claudin18.2 (CLDN18.2) is an emerging actionable target in many cancers, including PDAC.19 Therapeutics with various mechanisms of action are under investigation to exploit this target. IBI389 is a CLDN18.2×CD3 bispecific antibody evaluated in a phase 1 study (NCT05164458) in 64 previously-treated patients with CLDN18.2-positive PDAC.20 The ORR was 30.4% (95% CI, 13.2%-52.9%), and the disease control rate was 69.6% (95% CI, 47.1%-86.8%). TRAEs of grade 3 or greater were reported in 54.7% of patients. Treatment was discontinued in 4.7% of patients due to TRAEs. Cytokine release syndrome is an AE of particular concern with IBI389, with grade 1 or 2 events occurring in 51.6% of patients.
Zolbetuximab is an anti-CLDN18.2 monoclonal antibody being tested against several tumor types and will be assessed in combination with gemcitabine/nab-paclitaxel (GN) vs GN alone as first-line therapy in patients with metastatic pancreatic cancer in a phase 2 study (NCT03816163).21 The primary end point is overall survival.
Key References
4. Singhi AD, George B, Greenbowe JR, et al. Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomarkers. Gastroenterol. 2019;156(8):2242-2253.e4. doi:10.1053/j.gastro.2019.02.037
6. Davies KD, Aisner DL. Wake up and smell the fusions: single-modality molecular testing misses drivers. Clin Cancer Res. 2019;25(15):4586-4588. doi:10.1158/1078-0432.Ccr-19-1361
9. Schram AM, Goto K, Kim DW, et al. Efficacy of zenocutuzumab in NRG1 fusion-positive cancer. N Engl J Med. 2025;392(6):566-576. doi:10.1056/NEJMoa2405008
For FULL References List, visit https://www.gotoper.com/annual-oncology-meeting-25-nrg1-postref
CME Posttest Questions
1 A patient is referred to you with a new diagnosis of metastatic
pancreatic ductal adenocarcinoma (PDAC) with multiple liver
metastases. The patient is asymptomatic and has an ECOG perform-
ance status of 0. Which of the following would be the next best step?
A. Germline testing for inheritable pathogenic mutations
B. Germline testing and somatic tissue testing
C. Somatic tissue testing only
D. Initiate systemic treatment without further testing
2 Blockade of which of the following molecules is an active
treatment approach in tumors with NRG1 fusions?
A. EGFR
B. HER3
C. HER4
D. NRG1
3 A patient with metastatic PDAC is referred to you after disease
progression on FOLFIRINOX. No somatic testing was performed at the time of diagnosis. On further investigation, the tumor has no evidence
of KRAS mutations but has an ATP1B1-NRG1 fusion. Which of the following therapies would you choose at this time?
A. Gemcitabine plus nab-paclitaxel
B. Zenocutuzumab
C. Zenocutuzumab plus gemcitabine
D. Clinical trial of an NRG1 antibody
Claim Your CME Credit at
https://www.gotoper.com/annual-oncology-meeting-25-nrg1-postref
CME Provider Contact information
Physicians’ Education Resource®, LLC
2 Commerce Drive, Suite 110, Cranbury, NJ 08512
Toll-Free: 888-949-0045
Local: 609-378-3701
Fax: 609-257-0705
info@gotoper.com
