67-year-old Male with Prostatic Stromal Tumor of Uncertain Malignant Potential
STUMP: Elevated PSA, family history, risk factors, and clinical findings. What is the next step in this case? Experts weigh in.
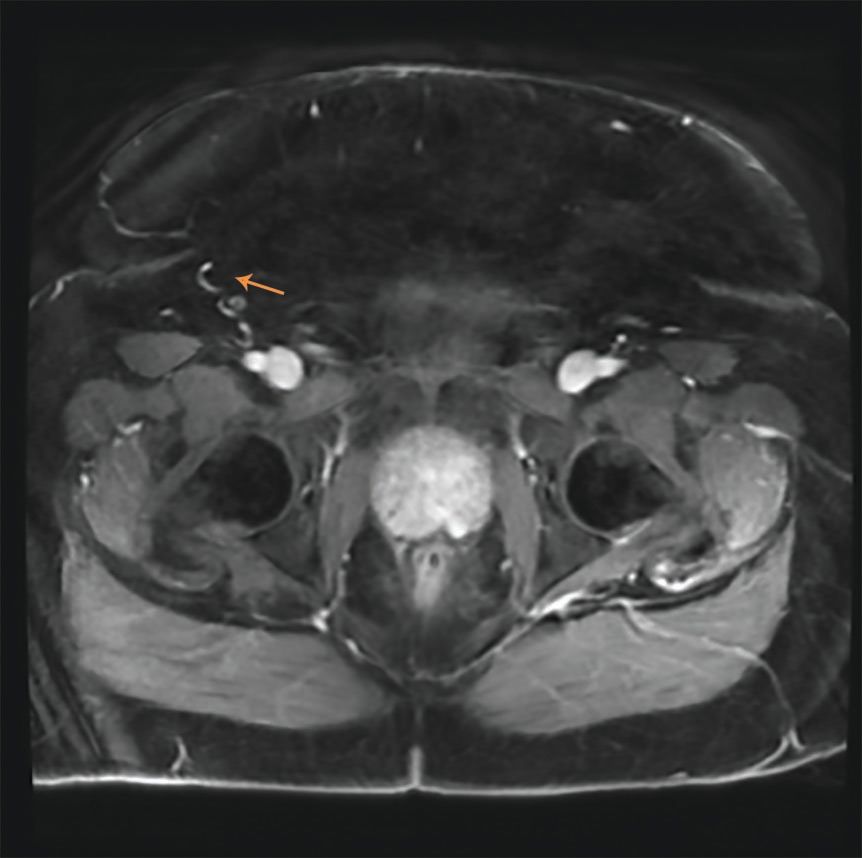
FIGURE 1: Axial T2 weighted image showing the 0.8x0.8 cm lesion (arrow).
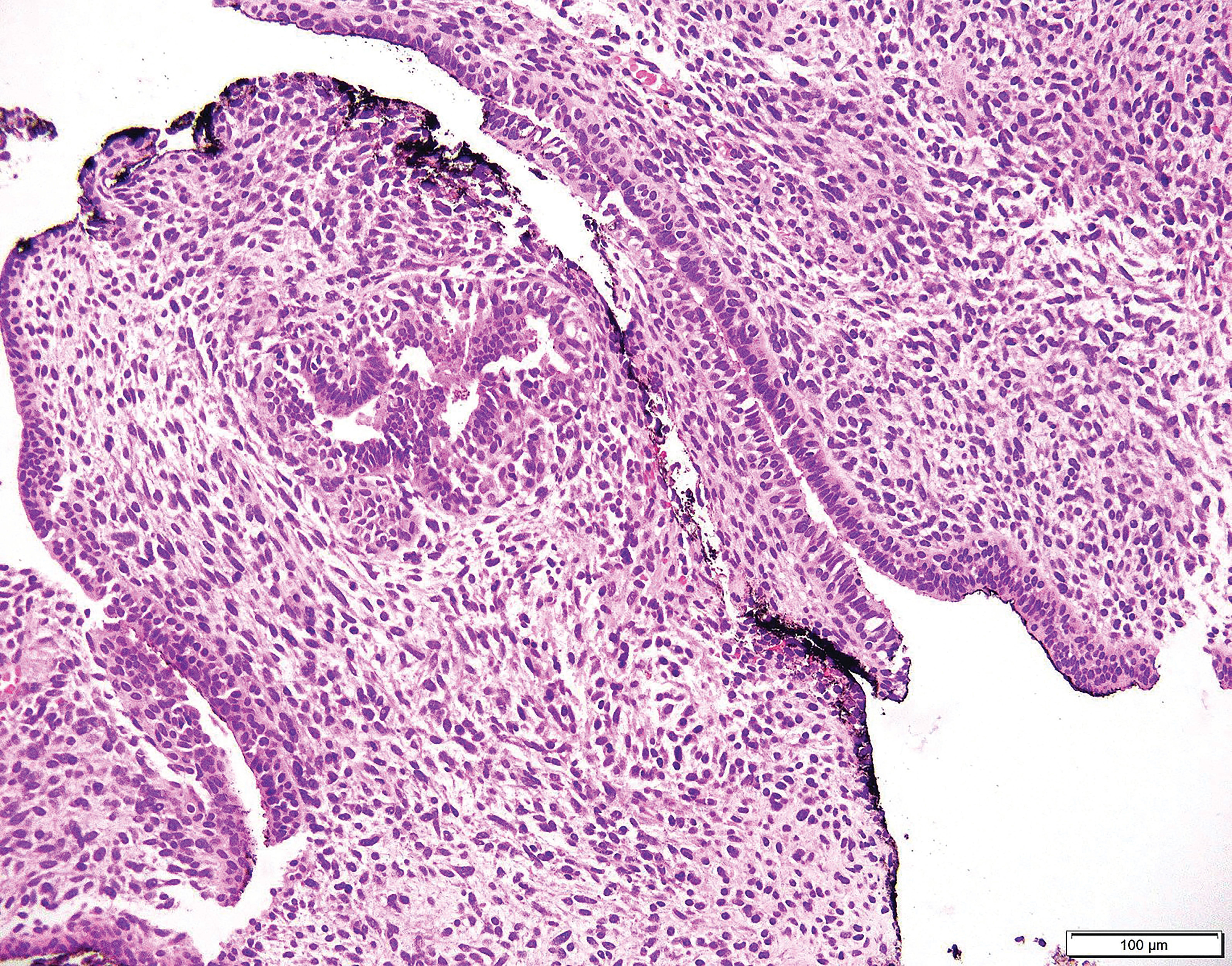
Micrograph of a prostatic stromal tumor of uncertain malignant potential, (STUMP), a lesion of the prostatic stroma that cannot be determined as either benign or malignant.
Luke Bidikov, MD

Paul Maroni, MD

Simon Kim, MD, MPH

THE CASE
Luke Bidikov, MD, Paul Maroni, MD, Simon Kim, MD, MPH, and E. David Crawford, MD
A 67-year-old African American male presents with an elevated prostate-specific antigen (PSA), which had risen from 6.6 ng/mL two years ago to 8.4 ng/mL four months ago. Two prior prostate biopsies were negative. A SelectMDx test one year ago showed a 50% risk of cancer, and a 23% risk of high-grade cancer. A recent prostate biopsy had a cellular myxoid cell neoplasm involving 20% of a core in the left-mid prostate. Tumor cells had 0.1% nuclear staining with Ki-67, no mitotic activity, and were focally positive for estrogen receptors. Due to the patient’s body habitus (body mass index 46), he was unable to obtain magnetic resonance imaging (MRI). Other medical problems include hypertension and type 2 diabetes. His father had prostate cancer. He has a 15-pack year smoking history but quit in 1984. Imaging is relevant for a CT with stable subcentimeter pulmonary nodules obtained six months prior. The diagnosis was likely benign tumor of the prostate but stromal tumor of uncertain malignant potential (STUMP) could not be excluded.
What is the next step in clinical management of this patient?A. Radical prostatectomy
B. Obtain chest CT and attempt MRI of the pelvis
C. Repeat prostate biopsy in six months
D. Focal cryoablation
E. Active surveillance with yearly PSA and biannual prostate needle biopsy
CORRECT ANSWER: C. Obtain chest CT and attempt MRI of the pelvis
Discussion
Mesenchymal neoplasms account for <1% of all cancer found in the prostate and are predominantly prostatic stromal proliferations. Other tumors found in prostate needle biopsy specimens include myofibroblastic proliferations, smooth muscle neoplasms, gastrointestinal stromal tumors, schwannomas, rhabdomyosarcomas, postradiation sarcomas, and mixed epithelial stromal tumors of the seminal vesicle.[1] The World Health Organization classifies prostatic stromal proliferations into two categories – STUMP and stromal sarcoma.[2]
STUMP tumors have much overlap with the stromal hyperplasia of benign prostatic hyperplasia (BPH) as well as variable degrees of epithelial proliferation.[3-5] Four histologic patterns have been described – a cellular spindled, eosinophilic pattern, a pattern with scattered atypical stromal cells, a phyllodes-like pattern, and a myxoid pattern[6] – with the recent addition of a “round-cell” pattern in a recent review.[7] They all have minimal mitotic activity and minimal necrosis.
STUMP have a range of clinical outcomes, and the histologic subtype has not been linked to a specific clinical outcome.[8] The clinical course of the disease is uncertain in part because there have been few published case series on STUMP.[6,8-9] The majority remain stable for years without intervention and do not recur if resected, some recur locally after repeated resections, and some develop (concurrently or subsequently) characteristics of stromal sarcoma, which can lead to widespread metastasis and death.[8,10-12] One case series of 18 patients encourages the use of the term “prostatic stromal hyperplasia with atypia” rather than STUMP, contending that the lesion is benign and has minimal risk of malignant transformation.[13]
Unfortunately, a prostate biopsy finding STUMP without any degree of sarcoma does not guarantee a stable disease course. In the largest case series, one STUMP preceded the development of stromal sarcoma by 9 years. In another patient, an initial needle biopsy yielded STUMP, but a subsequent radical prostatectomy (RP) showed STUMP and high-grade sarcoma. The clinical presentation is also non-specific. The mean age of presentation was 58 years. The most common presenting symptoms were lower urinary tract symptoms (LUTS), abnormal digital rectal exam, and hematuria, with a significant subset of patients biopsied due to a rising PSA.[8]
Treatment of STUMP ranges from observation to RP with chemoradiation, and outcomes are related to the degree of sarcoma present in the tissue. A case series of 21 patients with prostatic sarcoma (including rhabdomyosarcoma and leiomyosarcoma) who were treated with RP and chemoradiation yielded a survival rate of 38% at 5 years.[11] In a series of 23 patients with phyllodes tumor, 12 were treated with prostatectomy; of those, 4 had recurrent or persistent disease.[9] In one large case series, 50 patients were split between those with prostatic STUMP (n=36) and patients with partial or complete sarcoma (n=14). Of the pure STUMP tumors, 14 were watched without evidence of disease progression, 5 required repeated trans-urethral resections, and 14 underwent immediate RP without evidence of recurrence (3 were lost to follow up). The patients with sarcoma present all underwent RP. Of those, 4 had disease recurrence, 8 had no evidence of disease recurrence, and 2 were lost to follow up.[8] A different case series of 18 patients with “stromal hyperplasia with atypia” showed no development of sarcomatous differentiation or malignancy on follow-up.[13] These data suggest that RP should be seriously considered for patients with features of sarcoma at initial diagnosis. For all others, the benefits of RP as initial treatment are less clear; at the very least, these patients should undergo surveillance to watch for disease progression.
Very little is known about imaging STUMP. One case report of an MRI taken of a STUMP showed that the lesion had homogenous low-signal on T1 weighted image, but was diffusely heterogenous on T2 weighted image, with layered cysts located in areas of the tumor. Its appearance was quite different from adenocarcinoma of the prostate, but could not be readily differentiated from other prostatic tumors, including sarcomas – of note, the authors state sarcomas would not have a true cystic component, but if large would have areas of necrosis with a cystic appearance. In their case report, the mass was quite large (110 mL), and the extent of the lesion could not be accurately discerned given proximity to the seminal vesicles and anterior rectal wall. The patient ultimately underwent RP. There was no evidence of tumor extending beyond the prostate, and surgical pathology was consistent with STUMP.[14]
For our patient, we recommended a protocoled MRI and a repeat chest CT. A large lesion size or suspicion of extent into surrounding tissues on MRI would favor aggressive treatment, including RP and possible adjuvant chemoradiation. Enlarged pulmonary nodules on CT would raise concern for metastasis and might prompt biopsy. Conversely, a smaller, contained lesion on MRI and stable pulmonary nodules on CT would favor close monitoring of the lesion. A repeat prostate biopsy is a reasonable option, but the yield would be much higher if it were MRI-guided; likewise, focal cryoablation could be used in the future in the setting of a localized and better-defined lesion.
Our patient had no malignant features consistent with co-existing stromal sarcoma, and we thus did not immediately recommend RP. However, risk remained that the patient harbored malignant tissue not sampled by the needle biopsy, or that the STUMP could undergo malignant transformation over time. We had limited information on the size of the lesion – the patient had not undergone MRI at his outside hospital due to body habitus. Because of the concern for coexisting malignancy and lack of knowledge of tumor size, we did not recommend delayed follow-up.
Published case series are few and far between, and within those series, the histology and clinical course of the lesions are so varied that no protocol or guidelines can easily be applied to all STUMP tumors. We presented this information to the patient and his family. They were averse to undergoing a large operation as initial intervention and elected to gather further diagnostic information prior to initiating any treatment.
Outcome
An MRI was obtained which found a 0.8 x 0.8 cm lesion in the left medial posterior peripheral zone of the prostate, consistent with the location of the positive core biopsy. (Figure 1) There was focal bulging of the capsule overlying the lesion without evidence of definite extension. It had a circumscribed, homogenous appearance on T2 weighted image. The CT chest showed stable pulmonary nodules.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
Key Points
• STUMP is a rare tumor with a non-specific clinical presentation and varied histological characteristics.
• Pelvic imaging and review of pathology should be obtained to further characterize the tumor.
• Treatment is specific to the patient, and can include surveillance, local resection, or radical prostatectomy.
PERSPECTIVE

Comprehensive Assessment, Shared Decision Making Are Key
Edouard J. Trabulsi, MD and Thenappan Chandrasekar, MD
While prostate adenocarcinoma is the predominant malignant histology of the prostate, practicing clinicians, especially urologists and pathologists, should be aware of less common prostate histology. Prostatic stromal proliferations, classified by the WHO into either stromal tumor of uncertain malignant potential (STUMP) tumors or frank stromal sarcoma, are the predominant mesenchymal tumors of the prostate.
Though five histologic patterns have been described for STUMP tumors, it is important to note that all minimal mitotic activity, minimal necrosis and otherwise benign microscopic features. [1,2] STUMP tumors, even though they do not behave aggressively, are recognized as neoplasms, as some may occasionally demonstrate local recurrence after resection, be associated with local morbidity or have unrecognized malignant potential. Hence, the management of these lesions requires a thorough evaluation for evidence of malignant potential, especially undetected sarcoma, and subsequent management based on shared decision making with the patient.
Comprehensive assessment includes, but is not limited to, prostate biopsy (or biopsies, in cases of insufficient tissue), appropriate staging with cross-sectional imaging, and additional evaluations to ensure appropriate management for the patient. As the authors noted in this case, a prostate biopsy that identifies STUMP without any degree of sarcoma does not guarantee a stable disease course nor is it always consistent with final surgical pathology.[3] If sarcoma is suspected, but unable to be confirmed on tissue diagnosis, management should be tailored accordingly. Appropriate staging with cross-sectional imaging is critical, as findings of aggressive local disease or metastatic disease not appreciated on initial evaluation, can significantly impact treatment decision making. Along those lines, cross-sectional imaging, and additional evaluations, such as cystoscopy, cystogram, etc., may be important in cases of STUMP tumors with locally aggressive disease. For example, in a case at our center, the STUMP tumor presented as a 19 cm multilocular solid and cystic mass replacing the prostate gland with resultant left-sided bladder displacement. Subsequent evaluation with CT scan, MRI and cystoscopy enabled us to better plan for eventual surgical resection, including consideration of radical cystoprostastectomy.[4]
Finally, shared decision making is of the utmost important. As this is a rare condition, management options range from observation to more extensive surgical resection. A multi-disciplinary approach, in conjunction with colleagues from medical oncology and radiation oncology, is highly suggested. As sarcoma is often radiosensitive, radiation therapy should be considered as either neoadjuvant therapy or alternative to surgical excision. As the presence of sarcoma is the driving feature of oncologic outcomes,[3,5,8] surgical management should revolve about optimizing local control, minimizing risk of positive surgical margins, and, in our opinion, preclude focal therapy.
While the authors of this case report advocate for active surveillance, due to the lack of correlation between prostate biopsy and final surgical pathology, risk of sarcomatous differentiation, and lack of long-term surveillance data, it is our opinion that patients with STUMP tumors warrant active treatment, either with surgical excision or radiation therapy. However, if STUMP tumor without evidence of sarcoma is confirmed on final surgical pathology, close follow-up without adjuvant therapy appears to be safe.
FINANCIAL DISCLOSURE: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Sadimin ET, Epstein JI. Round cell pattern of prostatic stromal tumor of uncertain malignant potential: a subtle newly recognized variant. Hum Pathol. 2016;52:68-73.
2. Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am J Surg Pathol. 1998;22(2):148-62.
3. Herawi M, Epstein JI. Specialized stromal tumors of the prostate: a clinicopathologic study of 50 cases. Am J Surg Pathol. 2006;30(6):694-704.
4. Leong JY, Chandrasekar T, Sebastiano C, Rshaidat H, Steward JE, Trabulsi EJ. Prostatic Stromal Tumors of Uncertain Malignant Potential. Urology. 2019 Jun 26. [Epub ahead of print]
5 .Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M. D. Anderson Cancer Center Experience. J Urol. 2001;166(2):521-5.
6. Hossain D, Meiers I, Qian J, MacLennan GT, Bostwick DG. Prostatic stromal hyperplasia with atypia: follow-up study of 18 cases. Arch Pathol Lab Med. 2008;132(11):1729-33.
Dr. Trabulsi is a physician with the Department of Urology and the Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, Pennsylvania.
Dr. Chandrasekar is a physician with the Department of Urology and the Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, Pennsylvania.
Disclosures:
References:
1. McKenney JK. Mesenchymal tumors of the prostate. Mod Pathol. 2018;31:S133-42.
2. Cheville J, Algaba F, Epstein JI et al. Mesenchymal tumours. In: Moch H, Humphrey PA, Ulbright TM et al (eds). WHO Classification of Tumours of the Urinary System and Male Genital Organs. IARC Press: Lyon, France, 2016;pp 175–177.
3. Mokhtari M, Homayoun M, Yazdan Panah S. The prevalence of prostatic stromal tumor of uncertain malignant potential in specimens diagnosed as prostatic hyperplasia. Arch Iran Med. 2016;19:488-90.
4. Nagar M, Epstein JI. Epithelial proliferations in prostatic stromal tumors of uncertain malignant potential (STUMP). Am J Surg Pathol. 2011;35:898-903.
5. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20:148-58.
6. Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am J Surg Pathol. 1998;22:148-62.
7. Sadimin ET, Epstein JI. Round cell pattern of prostatic stromal tumor of uncertain malignant potential: a subtle newly recognized variant. Hum Pathol. 2016;52:68-73.
8. Herawi M, Epstein JI. Specialized stromal tumors of the prostate: a clinicopathologic study of 50 cases. Am J Surg Pathol. 2006;30:694-704.
9. Bostwick DG, Hossain D, Qian J, et al. Phyllodes tumor of the prostate: long-term followup study of 23 cases. J Urol. 2004;172:894-9.
10. Matsuyama S, Nohara T, Kawaguchi S, et al. Prostatic stromal tumor of uncertain malignant potential which was difficult to diagnose. Case Rep Urol. 2015;2015:879584.
11. Sexton WJ, Lance RE, Reyes AO, et al. Adult prostate sarcoma: the M. D. Anderson Cancer Center experience. J Urol. 2001;166:521-5.
12. Murer LM, Talmon GA. Stromal tumor of uncertain malignant potential of the prostate. Arch Pathol Lab Med. 2014;138:1542-5.
13. Hossain D, Meiers I, Qian J, MacLennan GT, Bostwick DG. Prostatic stromal hyperplasia with atypia: follow-up study of 18 cases. Arch Pathol Lab Med. 2008;132:1729-33.
14. Muglia VF, Saber G, Maggioni G Jr., Monteiro AJ. MRI findings of prostate stromal tumour of uncertain malignant potential: a case report. Br J Radiol. 2011;84:e194-6.

Prolaris in Practice: Guiding ADT Benefits, Clinical Application, and Expert Insights From ACRO 2025
April 15th 2025Steven E. Finkelstein, MD, DABR, FACRO discuses how Prolaris distinguishes itself from other genomic biomarker platforms by providing uniquely actionable clinical information that quantifies the absolute benefit of androgen deprivation therapy when added to radiation therapy, offering clinicians a more precise tool for personalizing prostate cancer treatment strategies.