Biliary Cancer: Current Multimodality Treatment and Future Directions
Clinicians discuss appropriate evaluation and choice of management strategies for cholangiocarcinoma.
Shaheed Merani, MD, PhD

Luciano M. Vargas, MD

FIGURE 2: Deregulated molecular pathways with potential targeted agents in biliary cancer..
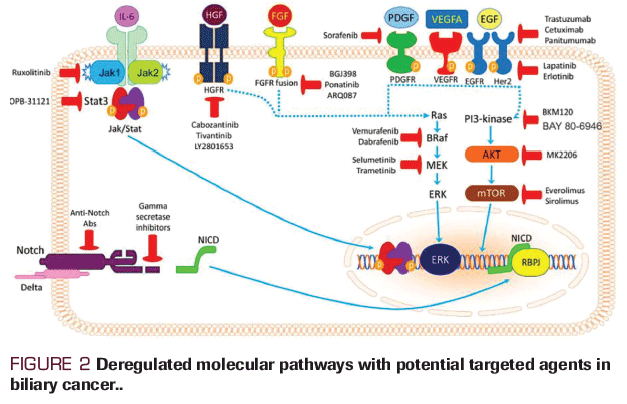
TABLE 1 :Currently Active Biliary Cancer Clinical Trials Supported by National Cancer Institute[56]
ABSTRACT: Biliary cancer is a highly aggressive malignancy arising from the biliary tree, with its incidence increasing steadily on a global level. Most biliary cancers are diagnosed in the advanced and metastatic stages due to the paucity of signs and symptoms in the early presentation. Only about one-third of the patients can be treated with curative intent with an overall median survival of less than 24 months for all-comers from the time of diagnosis. This fact and the poor results of the currently available local and systemic therapies, are responsible for the disappointing outcome of biliary cancer patients. There is an unmet need for novel therapeutic approaches. Surgery, the backbone of curative treatments for biliary cancer, is effective in early, completely-resectable stages or in combination with neoadjuvant or adjuvant chemotherapy and/or radiation therapy for locally advanced stages. Systemic therapies in unresectable and recurrent cases are associated with poor outcomes. The introduction of next-generation sequencing technologies has opened new horizons for a better understanding of the molecular basis of this cancer with potential identification and evaluation of new treatment options.
Introduction
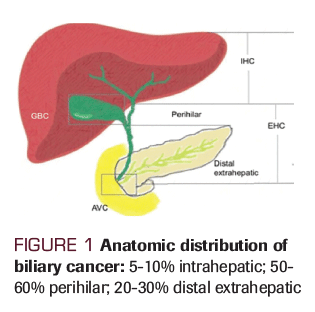
Biliary cancers are a heterogeneous group of tumors that originate from the epithelial lining cells of the small ducts within the liver and the main ducts of the biliary system extending into the gallbladder. Included under this designation are the cancers of gallbladder, intrahepatic and extrahepatic bile ducts, and variably, ampullary carcinoma. Extrahepatic ductal tumors are further divided into lesions that arise at the liver hilum, or at the extrahepatic ductal system. Inconsistent use of designations based on anatomical characteristics such as intrahepatic/extrahepatic or hilar has confounded the analysis of epidemiological trends of this cancer. The true incidence of biliary cancers is unknown due to the difficulty in establishing an accurate diagnosis. The incidence of cholangiocarcinoma in the western world is between 0.35 to 2 per 100,000 per year.[1] However, in China and Thailand, the incidence can be up to 40 times the rate observed in the western world in part due to liver fluke (ie, Opisthorchis viverrini) infestation.[2] Over the past 30 years the incidence of intrahepatic cholangiocarcinoma in the western world seems to be rising with an increase from 0.1 per 100,000 to 0.6 per 100,000 while the incidence of gallbladder cancer is on the decline.[3-7] This year, it is estimated that there will be 12,360 new cases of biliary cancer in the United States with 3,960 deaths.[8] About 50-60% of cholangiocarcinomas emerge in the bile ducts of the perihilar region; 20-30% emerge in the distal extrahepatic region; and 5- 10% emerge in the intrahepatic region.[9-11] (Figure 1).
Appropriate evaluation and choice of management for cholangiocarcinoma requires a multidisciplinary approach. Surgical resection remains the mainstay of curative intent therapy when tumors are resectable. However, most cases are multifocal at diagnosis and the tumor cannot be completely removed surgically making cure elusive. Palliative measures such as palliative resection, radiation therapy (e.g., brachytherapy or external-beam radiation therapy), or stenting procedures may maintain adequate biliary drainage and allow for improved quality of life. Chemotherapy and radiation therapy may potentially be useful in the neoadjuvant and adjuvant setting, while systemic therapy is needed for advanced disease presentation at diagnosis or for recurrent disease. Unfortunately, outcomes of traditional cytotoxic chemotherapies, whether as single agents or in combination, have been disappointing. Recent insights into the molecular biology of these heterogeneous tumors will hopefully lead to more effective systemic therapies.
In this article we focus on multidisciplinary management of biliary cancer in the preoperative, operative, postoperative, and advanced metastatic disease setting and provide a comprehensive list of currently available National Cancer Institute (NCI)-supported clinical trials for this disease.
Therapies
Neoadjuvant Therapy
Neoadjuvant chemotherapy may be helpful to select the patients who are most likely to benefit from surgical intervention, such as patients with jaundice, in patients with locally advanced gallbladder cancer, or in patients who have lymph node involvement. In a prospective study of locally advanced gallbladder cancer, 25 patients were given neoadjuvant chemoradiation and 15 patients were given neoadjuvant chemotherapy only. Six (two chemoradiotherapy and four neoadjuvant chemotherapy) patients were able to undergo curative intent resection. Four of them (66.7%) were alive at 18-month follow up.[11] In a retrospective study involving 74 patients with locally advanced or node positive gallbladder cancer who underwent neoadjuvant therapy, one-third of the patients were able to undergo resection, 45% of whom had definitive surgery. A median 51-month overall survival (OS) was associated with definitive resection compared with 11 months for the group who could not undergo resection.[12] Neoadjuvant chemotherapy should be considered in patients with evidence of locoregionally advanced disease. Chemotherapy regimens such as gemcitabine/cisplatin, gemcitabine/oxaliplatin (GEMOX), gemcitabine/capecitabine, capecitabine/cisplatin can be utilized. In a prospective study of 28 patients with locally advanced gallbladder cancer, R0 resection was possible in 14 patients. Following neoadjuvant treatment with gemcitabine and concurrent radiation therapy, 5-year survival in this group was 47%.[13] Although data on neoadjuvant chemoradiation remain limited to use in gallbladder cancer, this approach may also have a role in selected patients with cholangiocarcinoma. There is an unmet need for good quality, randomized trials in this setting.[14]
Surgical Therapy
The underlying surgical principle for the management of biliary cancers is to offer resection in the appropriate surgical candidate when R0 can be safely achieved. Surgical management of biliary tract cancers is tailored to the patient based on preoperative evaluation of both the patient and the tumor. Techniques for resection include hepatic and biliary resection with lymphadenectomy, paired with very careful peri-operative management. An individual approach is employed to balance the risk of surgical resection. This requires evaluating patient comorbidity, underlying liver disease, and tumor anatomy and biology. Since surgery for biliary cancers can cause morbidity, patients who have poor performance status or who have postoperative complications may not remain candidates for adjuvant therapy.
Surgical management of cholangiocarcinoma requires careful pre-operative evaluation of the location and extent of tumor. Pre-operative axial imaging with CT and MRI, as well as evaluation of biliary anatomy with endoscopic retrograde cholangiopancreatography (ERCP) can provide valuable information for operative planning. Anatomically, cholangiocarcinoma can arise from the extra-hepatic bile duct (either distal or hilar bile duct) or be more proximal and therefore completely intra-hepatic. Surgical resection is typically offered for patients without retro-pancreatic and para-celiac nodal metastasis, vascular involvement (of the portal vein and hepatic artery), or metastatic disease. Multifocal intra-hepatic tumor may be representative of metastatic disease rather than separate primary tumors and therefore is often considered a contraindication to surgical resection. Diagnostic laparoscopy or laparotomy for staging can be performed prior to resection. Complete resection of hilar cholangiocarcinoma most often requires major hepatectomy and bile duct resection with reconstruction along with hilar lymphadenectomy. Distal cholangiocarcinoma and ampullary cancers may be treated using similar surgical approach to pancreatic head tumors involving pancreaticoduodenectomy. Intra-hepatic cholangiocarcinoma often requires major hepatic resection; however, non-anatomical resection or segmental resections may be offered if negative margins can be achieved along with regional lymphadenectomy. [15]
Gallbladder adenocarcinoma is often diagnosed pathologically after cholecystectomy performed for presumed benign disease. Further resection to achieve negative margins and lymphadenectomy is often based on the depth of invasive carcinoma. For T1a tumors, cholecystectomy alone may be considered definitive resection, however T1b or greater tumors may require partial liver resection of the gallbladder bed, en bloc bile duct resection, or lymphadenectomy to clear local disease.[16]
Evaluation of the surgical patient with biliary cancer includes identification of comorbidities that would preclude major abdominal surgery, including evaluation of hepatic function and reserve. This can include axial imaging in the form of CT or MRI and assessment of hepatic volume including future liver remnant. Optimization of patients should include management of reversible obstructive jaundice with ERCP stent or percutaneous transhepatic biliary catheters prior to resection.[17]
Specific complications of surgery for biliary malignancies include bile leak, biliary stricture, positive resection margins or in situ disease at the margin, and post-hepatectomy liver failure. Due to the variability in location and extent of biliary cancers at the time of diagnosis, the rates of patients who undergo resection ranges from 56% to 91%.[18] Of those patients who do not undergo surgical resection, palliative interventions may be required. Typically, biliary decompression can be achieved by ERCP or percutaneous transhepatic biliary drains. In the recent era, the rates of palliative surgeries necessary for biliary cancers is lower due to improvements in pre-operative imaging and interventional procedures.[19]
Liver transplantation has been employed as a treatment option for select patients in the setting of biliary malignancy. Given the technical difficulty of achieving R0 resection of hilar cholangiocarcinoma, -short of complete hepatectomy-, liver transplantation has been explored. Some multicenter retrospective analyses demonstrate a survival benefit for select patients with hilar cholangiocarcinoma who received liver transplantation following neoadjuvant treatment.[20] Candidacy criteria for transplantation can include individuals with surgically unresectable tumors <3 cm, absence of nodal or metastatic disease, and those that have not undergone prior percutaneous biopsy. Transplantation is performed under specified protocols for hilar cholangiocarcinoma that typically include neoadjuvant external beam radiation and radiosensitizing chemotherapy, and variably brachytherapy or maintenance chemotherapy. Evaluation of outcome is limited by retrospective design of analyses, however one multicenter study found a dropout rate of 25% prior to transplantation, and a post-transplant recurrence free survival rate of 65% at 5-years.
Adjuvant Therapy
Complete resection remains the gold standard of treatment and offers the best chance of cure for patients with biliary tract cancers. However, 5-year OS rates even after complete resection range from 30% to 40% for perihilar lesions, 21% to 63% for intrahepatic cholangiocarcinoma,, and 20% to 54% for distal cholangiocarcinomas managed by pancreaticoduodenectomy.[21-24] Due to low incidence of biliary cancer there is a lack of prospective randomized trials to evaluate efficacy and safety of adjuvant therapy. Most of the retrospective trials with limited sample size and inclusion of heterogeneous patient population (combining gallbladder and cholangiocarcinoma patients) suggested a marginal benefit of chemoradiotherapy in reducing locoregional recurrence but an uncertain impact on survival.[25] Recurrence patterns differ by primary site: cholangiocarcinomas show a tendency to recur locally and provide a rationale for additional local therapy after definitive surgery while majority of gallbladder cancers have distant spread.[26]
A meta-analysis of 10 retrospective studies evaluating adjuvant radiotherapy after curative resection for extrahepatic cholangiocarcinomas demonstrated a significant benefit in OS in patients who received adjuvant radiotherapy.[27] On the contrary, a Surveillance, Epidemiology, and End Results (SEER) analysis of adjuvant radiotherapy failed to show a survival benefit; however, key data in this study, including margin status and the use of combined chemotherapy were not available through the SEER database.[28] In 2012, a systematic review and meta-analysis was undertaken to evaluate published data from 1960 to 2010. Twenty studies that compared surgery alone to surgery plus adjuvant therapy included 6712 patients, 1797 of whom received adjuvant therapy. In the overall analysis, adjuvant therapy was associated with a trend toward improvement in survival (odds ratio [OR], 0.74; P = .06). Chemotherapy and chemoradiation produced greater survival benefit than radiation alone. In patients with node-positive or margin positive disease, any adjuvant therapy had a significant benefit. Patients with R1 resection benefited from adjuvant radiotherapy and those with margin negative (R0) resection did not.[29]
The Southwest Oncology Group (SWOG) evaluated the use of adjuvant chemotherapy (gemcitabine and capecitabine) followed by chemoradiation (concurrent radiotherapy and capecitabine) for resected extrahepatic cholangiocarcinoma or gallbladder tumors. Out of 79 evaluable patients, 49 (62%) had extrahepatic cholangiocarcinoma. Twenty-five patients (32%) underwent an R1 resection. Two-year OS was 65% for all patients (67% and 60% for R0 and R1 resections, respectively). Median OS was 35 months, and only 14 patients developed a local recurrence. Grade 3 adverse events were observed in 52% and grade 4 in 11% of patients.[30]
The first randomized study evaluating the impact of adjuvant chemotherapy in biliary tract cancer was conducted by Takada et al[31] in 2002. Patients with pancreatic cancer (n = 158), extrahepatic biliary tract cancer (n = 118), gallbladder cancer (n = 112), and ampulla of Vater cancer (n = 48) were randomly assigned to adjuvant mitomycin plus 5-fluorouracil (5-FU) chemotherapy or to surgery alone. While patients with resected gallbladder cancer who had received mitomycin/FU had significantly better 5-year disease free survival compared with control (20.3% versus 11.6%) no difference was observed for all biliary tract cancers.[28] A decade later, the European Study Group for Pancreatic Cancer reported the results of ESPAC-3 trial which found a survival benefit for patients with ampullary cancers who received adjuvant 5-FU plus folinic acid or gemcitabine compared with observation after adjusting for prognostic variables.[32]
More recently, the PRODIGE 12-ACCORD 18-UNICANCER GI trial randomly assigned 196 patients to adjuvant GEMOX or to observation alone in resected biliary tract cancer (including all types). R1 resection rates were 13.8% in the GEMOX arm and 12.1% in the observation arm; lymph node involvement was 37.2% and 36.4%, respectively. No difference in the primary end point of relapse-free survival between the experimental and observation groups was observed (39% v 33% at 4 years; P = .31).[33] In contrast, in the BILCAP study which randomized 447 patients with resected biliary tract cancer to receive adjuvant capecitabine versus observation, median recurrence free survival was 24.4 versus 15.5 months, respectively and median OS was 51.1 months versus 36.4 months, respectively, favoring the capecitabine arm.[34] Finally, the phase III Japanese Bile Duct Cancer Adjuvant Trial (BCAT) which only included patients with extrahepatic cholangiocarcinoma and excluded patients with gallbladder cancer showed no difference in OS (median OS, 62.3 vs 63.8 months) or relapse-free survival (median relapse-free survival, 36.0 vs 39.9 months) between gemcitabine versus observation groups.[35]
Given the heterogeneity of tumor sites, variation in nodal and margin statuses, and nonuniform chemotherapy regimens, interpretation of all these data becomes very challenging. One may conclude that, with the ESPAC-3 data, there seems to be some benefit for adjuvant chemotherapy in ampullary cancers, and with the data from Takada et al. study some benefit for use of adjuvant chemotherapy for gallbladder cancers. Despite the paucity of level-1 evidence to support adjuvant therapy for biliary tract cancer, available guidelines at present support consideration of adjuvant chemotherapy, radiation therapy, and chemoradiation therapy, alone or in combination in most stages and subtypes of biliary cancer, particularly in node-positive and margin-positive disease. The optimal sequencing of these modalities and optimal chemotherapy regimens await larger randomized trials to address these questions.
Systemic Therapy for Advanced Metastatic Disease
Unfortunately, biliary cancer is often diagnosed at an advanced stage when resection is no longer possible. Even in patients with initial resectable disease, early postoperative recurrence makes systemic therapy necessary. The prognosis of patients with advanced biliary tract cancers is poor and median survival for those undergoing supportive care alone is short. Treatment options at this stage include gemcitabine plus cisplatin chemotherapy, fluoropyrimidine-based chemotherapy, pembrolizumab for patients with microsatellite instability high/deficient mismatch repair (MSI-H/dMMR) tumors, fluoropyrimidine-based chemoradiation, or palliative radiotherapy without chemotherapy and most certainly clinical trials. The randomized phase III clinical trial (ABC-02 Trial) which enrolled 410 patients with locally advanced or metastatic cholangiocarcinoma, gallbladder cancer, or ampullary cancer demonstrated the superiority of the combination of gemcitabine and cisplatin over gemcitabine monotherapy in terms of OS and PFS by 30%.[36] Other gemcitabine-based or fluoropyrimidine-based regimens with activity in this setting include GEMOX, oxaliplatin/5-FU/leucovorin, capecitabine/oxaliplatin, gemcitabine/albumin-bound paclitaxel, gemcitabine/cetuximab, and gemcitabine/oxaliplatin/5-FU.[37-42] In a phase II study combination of panitumumab/gemcitabine/irinotecan showed encouraging results.[43]
A standard regimen in the second-line setting has not yet been established. Agents with different mechanisms of action are needed. Fluoropyrimidines such as 5-FU, capecitabine, and S-1 are frequently used in clinical practice outside the USA. Several clinical trials are being conducted to evaluate the efficacy of molecularly targeted agents in the second-line or later settings. In a systematic review including 25 studies in advanced biliary cancer with data on 761 patients, the efficacy of second-line chemotherapy regimens was evaluated, and insufficient evidence was found to recommend a specific second-line regimen.[44]
Molecular Targets in Biliary Cancer
More than 90% of biliary tract cancers are well-differentiated, mucin-producing adenocarcinomas, whereas a very small proportion is squamous or small cell in origin. Novel therapeutic targets are desperately needed due to dismal overall prognosis for most cases of biliary tract cancer.[45,46] The information coming from molecular studies of biliary cancer has clearly shown that various human biliary cancer subsets characterized by heterogeneous molecular alterations exist. These alterations result in the activation of distinct signaling pathways which can be targeted. However, despite the identification of multiple targetable mutations (Figure 2.), due to the molecular complexity of biliary cancer the pioneering targeted approaches so far have produced less than satisfactory results.[47]
Biliary cancers share frequent genomic aberrations in the cell cycle regulators (specifically CDKN2B) and chromatin remodeling (ARID1A). Intrahepatic cholangiocarcinomas feature FGFR fusions, IDH1/2 substitutions, BRAF substitutions, and MET amplifications with a low KRAS mutational frequency.[48, 49] In a study of 153 biliary cancers (70 intrahepatic, 57 extrahepatic cholangiocarcinoma and 26 gallbladder carcinoma) the most frequently involved genes were KRAS (28%), TP53 (18%), ARID1A (12%), IDH1/2 (9%), PBRM1 (9%), BAP1(7%) and PIK3CA (7%). While IDH1/2 and BAP1 mutations were likely to be seen in intrahepatic cholangiocarcinom, KRAS and TP53 were more common in extrahepatic cholangiocarcinoma and gallbladder carcinoma.[49]
Somatic mutations in IDH1 and IDH2 cause a single amino acid change at a conserved arginine residue within the isocitrate binding site of IDH1 (R132) or IDH2 (R172, R140), causing reduced oxidative decarboxylation of isocitrate to ñ-ketoglutarate.[50] Rates of IDH1 mutations are higher than IDH2 (15%-23% vs 3%-4%, respectively) across reports of intrahepatic cholangiocarcinoma sequencing; studies evaluating IDH1 inhibitors in cholangiocarcinoma are ongoing.[51]
The EGFR pathway involvement has been shown in all of intrahepatic cholangiocarcinomas, half of extrahepatic cholangiocarcinomas, and more than a third of gallbladder cancers.[52] Erlotinib, a selective and reversible inhibitor of EGFR, has been studied in the management of advanced biliary tract cancer in several phase II studies with no definitive conclusions.[53] Monoclonal antibodies targeting EGFR in combination with chemotherapy have shown some success. Cetuximab in combination with GEMOX produced an impressive response rate of 63% with 8.3 months of progression-free survival (PFS) and 12.7 months of OS.[53] Because the expression of HER2 has been observed in about 26% of extrahepatic cholangiocarcinomas and 10% of gallbladder cancers[49], a phase II study of lapatinib, a dual HER2/EGFR inhibitor did not result in any response for patients with advanced biliary tract cancer.[54]
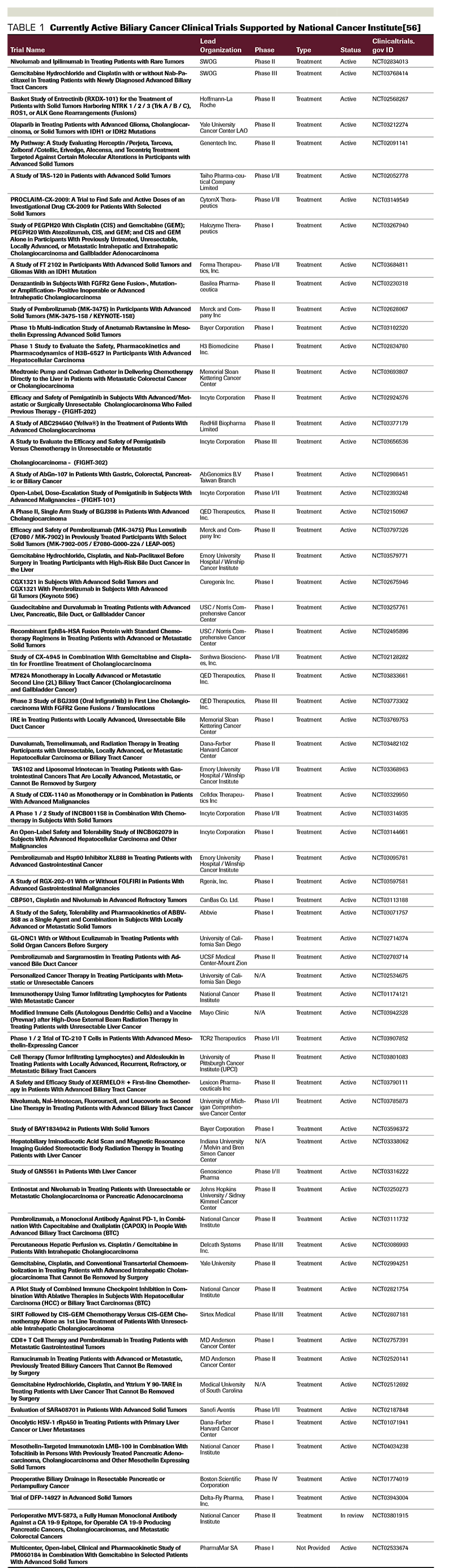
The MEK/ERK signaling pathway can be a therapeutic target for biliary tract cancers. A phase II study of the MEK1/2 inhibitor selumetinib in advanced biliary tract cancer resulted in a median PFS of 3.7 months and OS of 9.8 months, with a response rate of 12%.[55] Clinical studies using other MEK inhibitors are underway. A list of currently active National Cancer Institute-supported trials in biliary cancer are listed in Table 1.
Conclusion
Biliary cancer is a heterogeneous disease with a very poor prognosis. Many patients are diagnosed at an advanced stage when cure is not possible. It is crucial to implement a multidisciplinary team approach prior to initiation of any treatment. Complete resection, including liver transplantation in highly selected cases, is the only possibility for a curative therapy. Unfortunately, this is applicable only in a minority of cases. One of the main challenges is to find ways in increasing the number of resectable cases by expanding early diagnosis. A personalized diagnostic work-up and therapeutic approach must be applied by dedicated teams with multidisciplinary expertise. Participation in prospective clinical trials is the preferred option for all stages of disease (Table 1). Better understanding of the gene expression profile and mutational burden in biliary tract cancer will hopefully allow identification of new therapeutic targets and treatment options.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
Disclosures:
References:
1. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-7.
2. Sripa B, Kaewkes S, Sithithaworn P, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201.
3. West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006 94:1751-8.
4.Taylor-Robinson SD, Toledano MB, Arora S, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales1968-1998. Gut. 2001;48:816-20.
5. von Hahn T, Ciesek S, Wegener G, et al. Epidemiological trends in incidence and mortality of hepatobiliary cancers in Germany. Scand J Gastroenterol. 2011;46:1092-8.
6. Alvaro D, Crocetti E, Ferretti S, et al. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis. 2010;42:490-5.
7. Witjes CD, Karim-Kos HE, Visser O, et al. Intrahepatic cholangiocarcinoma in a low endemic area: rising incidence and improved survival. HPB (Oxford). 2012;14:777-81.
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
9. Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-80.
10. National Cancer Institute. (2019). Bile Duct Cancer (Cholangiocarcinoma) Treatment (PDQ). Available at https://www.ncbi.nlm.nih.gov/books/NBK65869/.
11.Agrawal S, Mohan L, Mourya C, Neyaz Z, Saxena R. Radiological downstaging with neoadjuvant therapy in unresectable gall bladder cancer cases. Asian Pac J Cancer Prev. 2016;17:2137-40.
12.Creasy JM, Goldman DA, Dudeja V, et al. Systemic chemotherapy combined with resection for locally advanced gall bladder carcinoma: surgical and survival outcomes. J Am Coll Surg. 2017; 224:906-16.
13. Engineer R, Goel M, Chopra S, et al. Neoadjuvant chemoradiation followed by surgery for locally advanced gallbladder cancers: a new paradigm. Ann Surg Oncol. 2016;23:3009-15.
14. Skipworth JR, Olde Damink SW, Imber C, et al. Review article: surgical, neoâ€Âadjuvant and adjuvant management strategies in biliary tract cancer. Aliment Pharmacol Ther. 2011;34:1063-78.
15. Seyama Y,Makuuchi M. Current surgical treatment for bile duct cancer. Wolrd J Gastroenterol 2007;13:1505-15.
16. D̢۪Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16:806-16.
17. Boulay BR, Birg A. Malignant biliary obstruction: from palliation to treatment. World J Gastrointest Oncol. 2016;8:498-508.
18. Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996; 224:463-75.
19. Buettner S, Wilson A, Margonis GA, et al. Assessing trends in palliative surgery for extrahepatic biliary malignancies: a 15-Year multicenter study. J Gastrointest Surg. 2016; 20:1444-52.
20.Sapisochin G, Fernandez de Sevilla E, Echeverri J, Charco R. Liver transplantation for cholangiocarcinoma: Current status and new insights. World J Hepatol. 2015;7:2396-403.
21. Darwish Murad S, Kim WR, Harnois DM et al. Efficacy of neoadjuvant chemoradition followed by liver transplantation for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88-98e. doi:10.1053/j.gastro.2012.04.008
22. Carpizo DR, D̢۪Angelica M. Management and extent of resection for intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2009;18:289-305, viii-ix.
23. Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004; 24:189-99.
24. Fong Y, Blumgart LH, Lin E, Fortner JG, Brennan MF. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996; 83:1712-15.
25. Cereda S, Belli C, Reni M. Adjuvant treatment in biliary tract cancer: to treat or not to treat? World J Gastroenterol. 2012;18:2591-6.
26. Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689-700.
27. Bonet Beltrán M, Allal AS, Gich I, Sole JM, Carrio I. Is adjuvant radiotherapy needed after curative resection of extrahepatic biliary tract cancers? A systematic review with a meta-analysis of observational studies. Cancer Treat Rev. 2012;38:111-9.
28. Vern-Gross TZ, Shivnani AT, Chen K, et al. Survival outcomes in resected extrahepatic cholangiocarcinoma: effect of adjuvant radiotherapy in a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys. 2011;81:189-98.
29. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012 ;30:1934-40.
30. Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: a phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol. 2015; 33:2617-22.
31. Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685-95.
32. Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147-56.
33. Edeline J, Bonnetain F, Phelip JM, et al. Gemox versus surveillance following surgery of localized biliary tract cancer: results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. J Clin Oncol. 2017;35(suppl; abstr 225)
34. Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. J Clin Oncol. 2017;35(suppl; abstr 4006)
35. Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105:192-202.
36. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273-81.
37. André T, Reyes-Vidal JM, Fartoux L, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer. 2008;99:862-7.
38. Sahai V, Catalano PJ, Zalupski MM, et al. Nab-Paclitaxel and gemcitabine as first-line treatment of advanced or metastatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018; 4:1707-12.
39. Nehls O, Klump B, Arkenau HT, et al. Oxaliplatin, fluorouracil and leucovorin for advanced biliary system adenocarcinomas: a prospective phase II trial. Br J Cancer. 2002;87:702-4.
40. Nehls O, Oettle H, Hartmann JT, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98:309-15.
41. Borbath I, Ceratti A, Verslype C, et al. Combination of gemcitabine and cetuximab in patients with advanced cholangiocarcinoma: a phase II study of the Belgian Group of Digestive Oncology. Ann Oncol. 2013; 24:2824-9.
42. Wagner AD, Buechner-Steudel P, Moehler M, et al. Gemcitabine, oxaliplatin and 5-FU in advanced bile duct and gallbladder carcinoma: two parallel, multicentre phase-II trials. Br J Cancer. 2009;101:1846-52.
43. Sohal DP, Mykulowycz K, Uehara T, et al. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann Oncol. 2013;24:3061-5.
44. Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014; 25:2328-38.
45. Marino D, Leone F, Cavalloni G, Cagnazzo C, Aglietta M. Biliary tract carcinomas: from chemotherapy to targeted therapy. Crit Rev Oncol Hematol. 2013;85:136-48.
46. Zhu AX, Hezel AF. Development of molecularly targeted therapies in biliary tract cancers: reassessing the challenges and opportunities. Hepatology. 2011;53:695-704.
47. Simile MM, Bagella P, Vidili G, et al. targeted therapies in cholangiocarcinoma: emerging evidence from clinical trials. Medicina (Kaunas). 2019 55(2). Pii:E42.
48. Ross JS, Wang K, Catenacci DVT, et al. Comprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and genomic alterations. J Clin Oncol. 2015; 33(suppl; abstr 4009).
49. Simbolo M, Fassan M, Ruzzenente A, et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget. 2014;5:2839-52.
50. Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72-9.
51.Bridgewater JA, Goodman KA, Kalyan A, Mulcahy MF. Biliary tr act cancer: epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Educ Book. 2016;35:e194-203.
52. Pignochino Y, Sarotto I, Peraldo-Neia C, et al. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer. 2010;10:631.
53. Gruenberger B, Schueller J, Heubrandtner U, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol. 2010;11:1142-8.
54. Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009; 64:777-83.
55. Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol. 2011; 29:2357-63.
56. National Cancer Institute. Find NCI-Supported Clinical Trials. Available at: https://www.cancer.gov/about-cancer/treatment/clinical-trials/search Accessed September 1, 2019.
