Practical Dosing Considerations for Venetoclax
With FDA-approved indications for both chronic lymphocytic leukemia and acute myeloid leukemia, and continued investigation within multiple disease states, venetoclax has become an exciting oral targeted therapy among oncologists.
TABLE 1: Timing of Venetoclax Ramp-Up in CLL Combination Regimens
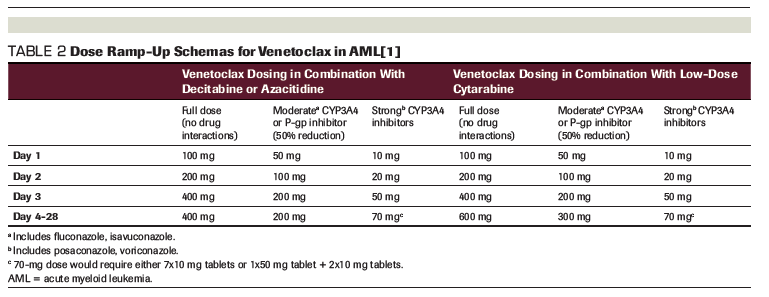
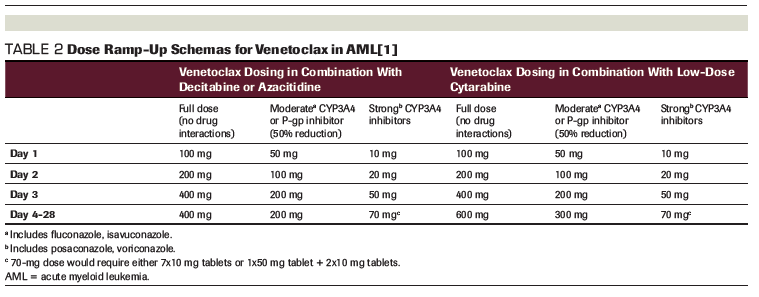
TABLE 2: Dose Ramp-Up Schemas for Venetoclax in AML[1]
With US Food and Drug Administration (FDA)-approved indications for both chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML), and continued investigation within multiple disease states, venetoclax has become an exciting oral targeted therapy among oncologists. Venetoclax is a small molecule inhibitor of the antiapoptotic protein BCL-2 and was first approved in the spring of 2016 as monotherapy in relapsed or refractory CLL with deletion 17p.[1] Initial attention to venetoclax focused not only on the clinical benefit of this agent but also on reported fatalities related to tumor lysis syndrome (TLS) seen in early studies. Much of the provider and patient education for venetoclax focuses on the risk for TLS and the importance of the ramp-up dosing packaged in a user-friendly manner in the starter pack. Various reminders for adequate hydration are prominent within the starter pack, and the manufacturer even has a 28-oz water bottle that can be obtained for patients. Detailed risk assessment and instructions for adjunctive supportive agents for prevention of TLS (eg, allopurinol, rasburicase, fluids) are outlined clearly in the prescribing information. Aside from being cognizant of TLS risk, there are a few additional practical considerations to review in prescribing this specialty medication.
A factor that can complicate patient counseling and medication prescribing is the recommended timing and duration of venetoclax in combination regimens. For example, when using the combination of venetoclax and obinutuzumab in the frontline setting for CLL, therapy begins with the CD20-directed monoclonal antibody (Table 1).[2] Patients should receive obinutuzumab on days 1, 2, 8, and 15 before starting their venetoclax ramp-up on day 21 of the first 28-day cycle.[1] Furthermore, this combination has allowed for a time-limited upfront treatment approach in which, as well as a finite period of 6 months for obinutuzumab, venetoclax is stopped after completing 12 months of therapy. This is different than the current recommendation for ibrutinib, the other breakthrough targeted agent, in the frontline setting. Conversely, when following the protocol used in the MURANO study, in which venetoclax is combined with rituximab in the relapsed or refractory setting, patients should complete the venetoclax ramp-up before the first infusion of rituximab and continue for 2 years, or until progression or unacceptable toxicity occurs (Table 1).[3]
In contrast, when used in combination therapies for AML, venetoclax should begin at the same time as the selected partner medication: decitabine, azacitidine, or low-dose subcutaneous cytarabine.[1] Another key difference when prescribing venetoclax in this setting is much faster ramp-up schemas over days instead of weeks without worrisome rates of TLS.[4,5] It should be noted that a target dose of venetoclax 400 mg daily is used when venetoclax is combined with the hypomethylating agents decitabine or azacitidine, but a higher target dose (600 mg) is recommended when patients receive a combination of venetoclax and low-dose subcutaneous cytarabine.[1] Prescribers should pay special attention to the implications of drug–drug interactions in the AML population, especially for patients with long-standing actual or functional neutropenia who may be receiving prophylactic azole antifungals. A useful summary of recommendations for initial dose titration is presented in Table 2.
Adding to the complexity of dosing are the available tablet strengths of venetoclax: 10 mg, 50 mg, and 100 mg.[1] Because the manufacturer’s starter pack was developed with the slower CLL dose ramp-up, it is not the ideal prescription product for patients initiating venetoclax combination therapy for AML. In patients without identified interacting concomitant medications, accelerated ramp-up allows for use of the 100-mg tablet strength and instructions to take 1, 2, 4, and potentially up to 6 tablets daily, with the highest pill burden required to achieve the target dose in combination with cytarabine only. Dose-adjusted ramp-up options for patients on concomitant moderate or strong cytochrome P450 3A4 (CYP3A4) or P-glycoprotein (P-gp) inhibitors require more complex prescribing that could lead to errors or confusion. In our practice, when dealing with moderate CYP3A4 or P-gp inhibitors, we have been inclined to start with venetoclax 100 mg by mouth daily for 2 days to eliminate the need for a separate prescription for a single 50-mg tablet, especially in patients for whom TLS is not of much concern. Similarly, in those on strong CYP3A4 inhibitors, our leukemia group has made the decision to consider use of a slightly higher target dose of 100 mg starting on day 4 and continuing for the duration of therapy unless there is a need to hold or reduce venetoclax. Consideration for this alternative dosing should be thought through with respect to the risk of side effects in cases in which use of a single-strength tablet for the dosing of venetoclax may reduce patient confusion and potentially pill burden.
Based on the summary of dosing presented here, it is essential to have a handle on the variations of how this drug is used across disease states. Optimal dosing, ramp-up schedules, and duration of therapy can be a cause for confusion and complicate prescribing. With the possibility of new FDA indications, current off-label utilization based on emerging data, and investigational combinations comes the potential for more nuances to arise with the use of venetoclax in practice. Although much of the information outlined in this brief review is covered within the prescribing information, multidisciplinary collaboration for venetoclax dosing has, at least in my practice, ensured safe, effective, and patient-friendly application of this therapeutic option.
FINANCIAL DISCLOSURE:The author has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
PERSPECTIVE

Venetoclax is Worth the Hassle
Kerry A Rogers, MD
Venetoclax is not an easy drug to start. In CLL there is a 5 week dose ramp-up scheme which requires outpatient or in hospital monitoring for tumor lysis based on disease burden. This necessitates a multidisciplinary approach and can be difficult to implement in practice. Adding to the complexity, the approved anti-CD20 monoclonal antibody for combination and timing of starting this relative to venetoclax is different in the treatment-naïve or relapsed/refractory settings.[1,2] Similarly, AML has different dosing schemes depending on the combination agent and the choice of azole antifungal prophylaxis.[3,4] Safely starting venetoclax places a burden on the healthcare team to ensure correct dosing and adequate monitoring for tumor lysis. It also places a burden on patients due to frequent clinic visits and in some cases hospital stays. When considering this effort, it is important to also consider the benefit for our patients.
In CLL venetoclax is superior to standard chemoimmunotherapy in terms of progression-free survival and has replaced it as a standard of care. This was demonstrated in the randomized phase 3 MURANO trial where it was compared to bendamustine and rituximab in relapsed/refractory CLL patients.1 Similarly, in the recently reported CLL14 study which randomized previously untreated CLL patients with coexisting medical illnesses to either venetoclax and obinutuzumab or chlorambucil and obinutuzumab, venetoclax and obinutuzumab provided improved progression free survival.[2] Additionally, venetoclax has demonstrated efficacy in patients progressing after ibrutinib.[5] This is a population where other approved therapies are of limited benefit and venetoclax dramatically increases survival expectations.
The impact of venetoclax on the treatment of AML is also substantial. Both approved combinations offer effective therapy for patients who are not suitable for standard induction chemotherapy. Both also have a more rapid time to recovery of peripheral blood counts than a hypomethylating agent or low dose cytarabine treatment, which decreases transfusion needs and risk of infection.[3,4] Importantly, responses were seen in AML patients with adverse risk disease, a population with limited alternative treatment options and inferior responses to traditional cytotoxic chemotherapy.
In addition to the approved indications for venetoclax which have positively impacted both diseases, the potential for highly-effective venetoclax combinations will only expand the uses for this drug. It is important to realize that the work required to safety use venetoclax is absolutely worth it based on the value venetoclax has for our patients in terms of disease outcomes.
Disclosures:
References:
1. Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med. 2018;378(12):1107-1120.
2. Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N Engl J Med. 2019;380(23):2225-2236.
3. DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17.
4. Wei AH, Strickland SA, Jr., Hou JZ, et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J Clin Oncol. 2019;37(15):1277-1284.
5. Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19(1):65-75.
