Adding Capecitabine to Gemcitabine Offers No Overall Survival Advantage, Though Could Benefit Some With Advanced Pancreatic Cancer
BASEL, SWITZERLAND-The combination of gemcitabine(Gemzar)/capecitabine (Xeloda)(GemCap) failed to prolong survivalmore than gemcitabine (Gem) alone,
BASEL, SWITZERLAND-The combination of gemcitabine(Gemzar)/capecitabine (Xeloda)(GemCap) failed to prolong survivalmore than gemcitabine (Gem) alone,although the combination was welltolerated and easily administered, RichardHerrmann, MD, of the UniversityHospital, Basel, Switzerland, reported(abstract LBA4010). The studywas supported in part by Hoffmann-LaRoche and Eli Lilly."Patients with good performancestatus achieved a significant gain inmedian survival of 2.6 months, or 35%,when treated with GemCap. Therefore,in patients with advanced cancerof the pancreas and good performancestatus, GemCap seems to be a goodalternative to single-agent treatment,"Dr. Herrmann said.A Multi-institutional StudyDr. Herrmann reported on a multiinstitutionalstudy of patients with histologicor cytologic proof of primaryinoperable or metastatic adenocarcinomaof the pancreas, a Karnofskyperformance score (KPS) ≥ 60%, andno previous chemotherapy. Stratificationfactors included locally advanced/metastatic disease, absence/presenceof pain, institution, and a KPS of 60-80 vs 90-100.The primary endpoint was overallsurvival, and secondary endpointswere quality of life, clinical benefit,objective tumor response (accordingto RECIST criteria), duration of response,time to disease progression,and toxicity. The investigators hypothesizedthat the median overall survivalwould be 5 months with gemcitabineand 7 months for combination gemcitabine/capecitabine.
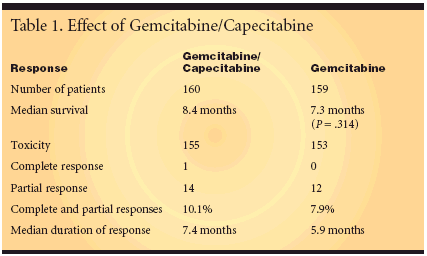
Better Results With GoodPerformance StatusA total of 319 patients were randomizedto receive GemCap (n = 160)or Gem (n = 159). The treatment protocolfollows: GemCap-gemcitabine(1,000 mg/m2 IV on days 1 and 8) andcapecitabine (650 mg/m2 twice dailyon days 1-14 every 3 weeks) or gemcitabine(1,000 mg/m2 weekly x 7, then 1week rest, and then weekly x 3 every 4weeks). Treatment continued for 6months or until disease progression.Dr. Herrmann cautioned, "Please notethat there are other versions of thisregimen using higher doses of capecitabineand different schedules." Threehundred patients were evaluable forresponse (148 on GemCap, and 152on Gem).The median overall survival was8.4 months for the GemCap armand 7.3 months for the Gem group(P = .314). Dr. Herrmann pointed outthat survival in the gemcitabine-onlygroup was 2 months longer thanexpected, rendering the differencebetween gemcitabine and combinedgemcitabine/capecitabine insignifi-cant (Table 1).The confirmed response rate was10.1% for GemCap vs 7.9% for Gem.The median duration of response was7.4 months for GemCap vs 5.9 monthsfor Gem, and the median time to diseaseprogression was 4.8 months forGemCap vs 4.0 months for Gem.A multivariate Cox regression ofoverall survival showed that in patientswith a KPS ≥ 90, those treatedwith GemCap had a significantly longermedian overall survival of 10.1months vs 7.5 months for Gem (P =.024)."Overall, GemCap does not improveoverall survival as compared tothe present standard gemcitabine.However (as indicated by subgroupanalysis), in patients with a goodperformance status, a significantimprovement in median overall survivalof 2.6 months can be achieved,"Dr. Herrmann said.In discussing this trial EileenO"Reilly, MD (Memorial Sloan-KetteringCancer Center, New York) said,"For now, the control arm of futuretrials should remain single-agent gemcitabine."