Adjuvant Endocrine Therapy for Breast Cancer: How Long Is Long Enough?
New evidence now supports improved recurrence and breast cancer mortality outcomes with continued tamoxifen for up to 10 years in women of any age, and such long-duration therapy is especially important for women who remain premenopausal after their first 5 years of tamoxifen.
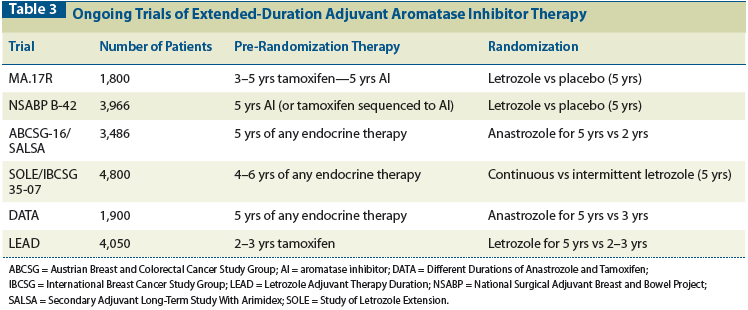
Table 1: Adjuvant Trials of Extended Tamoxifen Therapy Beyond 5 Years
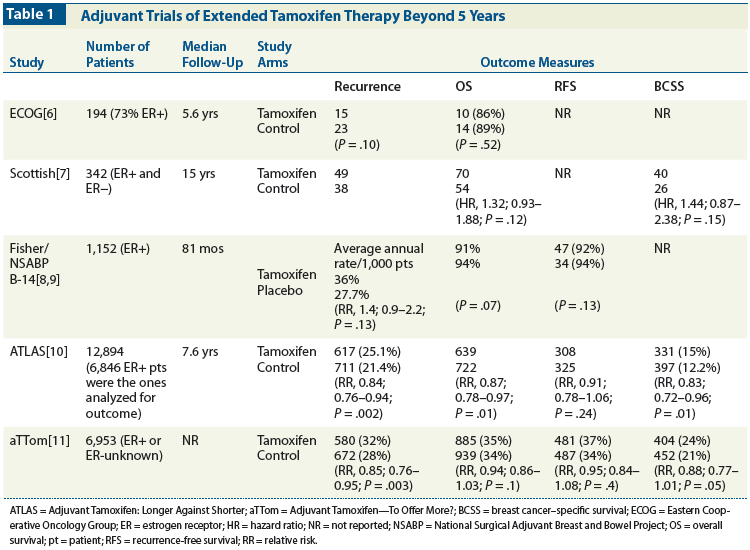
Table 2: Adjuvant Trials of Extended Aromatase Inhibitor Therapy Beyond 5 Years of Initial Endocrine Therapy
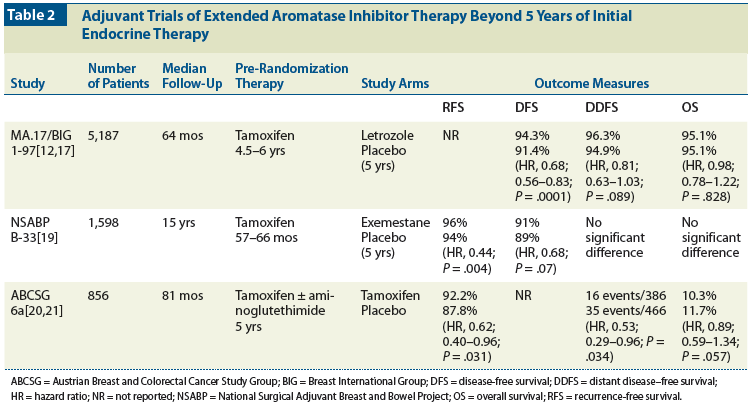
Table 3: Ongoing Trials of Extended-Duration Adjuvant Aromatase Inhibitor Therapy
Although women with early-stage hormone receptor–positive breast cancer have low recurrence rates initially, they have a constant and unrelenting risk of relapse that extends up to 15 years despite the use of adjuvant therapy. Increasing evidence supports the use of extended endocrine therapy with either tamoxifen or an aromatase inhibitor (AI) after 5 years of initial adjuvant tamoxifen to reduce breast cancer recurrence and mortality. However, the optimal total duration of AI therapy, as well as the ideal timing of sequencing from tamoxifen to an AI, is still unclear. Potential strategies differ depending on a woman’s menopausal status at the time of her initial diagnosis. Individual patient clinical factors and preferences can help with decision making until further data emerge on prolonged AI use and on potential biomarkers that can be used to tailor adjuvant endocrine treatment.
Introduction
Women with early-stage breast cancer are burdened with ongoing risk of relapse.[1] Specifically, patients with hormone receptor–positive breast cancer initially have lower rates of recurrence compared with women who have hormone receptor–negative disease, but their constant rate of relapse over time results in higher rates of late recurrence, even with systemic adjuvant therapy.[2,3] For this reason, clinical investigation has focused on the optimal duration of adjuvant endocrine therapy.
The most robust evidence for the benefit of adjuvant endocrine therapy for both pre- and postmenopausal women comes from the extensive evaluation of tamoxifen. Five years of tamoxifen therapy became the backbone of adjuvant hormonal therapy based on a series of randomized trials. Although significant reductions in recurrence and breast cancer death rates were seen with only 1 to 2 years of tamoxifen use, there were comparatively greater-and highly significant-improvements in recurrence rates (2P < .00001) and breast cancer mortality rates (2P = .0001) with 5 years of tamoxifen, with most of the effect on recurrence seen during years 1 through 5, and the mortality benefit seen mainly after 5 years.[4]
In the most recent Oxford overview of women with estrogen receptor (ER)-positive breast cancer, which included 15 years of follow-up, use of 5 years of adjuvant tamoxifen (compared with no therapy) resulted in a halving of recurrence rates during years 0 through 4, and a reduction by one-third in years 5 through 9.[5] This translated to absolute reductions in the risk of breast cancer recurrence and breast cancer mortality of 13% and 9%, respectively. The benefits of tamoxifen were seen independent of age, chemotherapy use, menopausal status, and nodal status. The risk of contralateral breast cancer was also lowered by 38% over all time periods, independent of age. Tamoxifen use increased the risk of uterine cancer in an age-dependent manner, with little absolute risk seen in women younger than 54 years but an absolute excess risk of 2.6% for women aged 55 to 69 years (a 3.8% 15-year incidence seen in the tamoxifen group vs a 1.1% incidence in the control group). There was a slightly increased thromboembolic risk, but this too was most apparent in older women.
Because of the concern about persistent risk of recurrence, three subsequent trials, the Eastern Cooperative Oncology Group (ECOG) trial, the Scottish trial, and the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 extension study, looked at the use of tamoxifen beyond 5 years (Table 1).[6-9] They included 1,588 patients in total, and in aggregate did not show that 10 years of tamoxifen therapy had greater benefit than 5 years of tamoxifen therapy. The largest of these studies, the NSABP B-14 extension study, randomly assigned more than 1,100 patients to therapy with either placebo or tamoxifen after completion of 5 years of adjuvant tamoxifen therapy.[8,9] Through 7 years of follow-up, a slight advantage was actually seen for patients who discontinued tamoxifen, compared with those who continued to receive it (disease-free survival [DFS], 82% vs 78%, respectively; P = .03), (recurrence-free survival [RFS], 94% vs 92%, respectively; P = .13). Moreover, there was concern about cumulative toxicity with ongoing tamoxifen use, due to an endometrial cancer rate in the tamoxifen arm of 2.1%, compared with 1.1% in the placebo arm (relative risk [RR], 2.0; 95% confidence interval [CI], 0.7–6.6). This led to a clinical alert from the US National Cancer Institute recommending that 5 years of adjuvant tamoxifen therapy be the standard of care for all women of any age with invasive HR-positive breast cancer.
Newer data from two larger studies, the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial and the Adjuvant Tamoxifen-To Offer More? (aTTom) study (see Table 1), now support longer-term tamoxifen use (for up to 10 years).[10,11] Additionally, in the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) MA.17/Breast International Group (BIG) 1-97 trial, extended adjuvant therapy with letrozole after 5 years of tamoxifen significantly reduced recurrence risk in patients with early-stage breast cancer, compared with placebo, supporting the use of extended aromatase inhibitor (AI) therapy after tamoxifen.[12] However, uncertainty remains regarding the best total duration of AI therapy, since there are no direct comparisons of various AI dosing strategies-such as initial, sequential, and extended adjuvant-nor are there comparisons of these treatments with extended tamoxifen therapy. Despite these gaps in our knowledge, a definitive signal has emerged in favor of extended endocrine therapy beyond 5 years for women with early-stage breast cancer. A recent meta-analysis of eight published randomized trials showed that for ER-positive breast cancer, extended adjuvant therapy after 5 years of tamoxifen yielded significant improvements in overall survival (odds ratio [OR], 0.89; 95% CI, 0.80–0.99; P = .03), breast cancer–specific survival (BCSS) (OR, 0.78; 95% CI, 0.69–0.90; P = .0003), and RFS (OR, 0.72; 95% CI, 0.56–0.92; P = .01). This translated to a 36% reduction in local regional relapse and a 13% reduction in distant relapse. Extended adjuvant endocrine therapy, compared with 5 years of tamoxifen alone, reduced the risk of death by 10% and the risk of relapse by 30% in patients with ER-positive breast cancer.[13]
The ATLAS and aTTom trials (see Table 1) have now reported results for extended treatment with 10 years of adjuvant tamoxifen vs 5 years.[10,11] Both trials randomized women with early breast cancer who had completed 5 years of tamoxifen treatment to either continuing tamoxifen to 10 years, or stopping at 5 years. Taken together, these trials report outcomes of 18,599 women with early-stage breast cancer. In the ATLAS trial, continued tamoxifen in ER-positive patients (n = 6,846) resulted in an absolute reduction in breast cancer recurrence of 3.7% (21.4% for women allocated to continuation vs 25.1% for controls; P = .002). Breast cancer mortality (331 deaths vs 397 deaths; P = .01) and overall mortality (639 deaths vs 722 deaths; P = .01) were also significantly reduced with 10 years of tamoxifen. The effects of continued tamoxifen were time-dependent, with minor reductions in recurrence rates seen during years 5 through 9 (RR, 0.90; 95% CI, 0.79–1.02; P = .10), but a robust carryover benefit seen during late follow-up (after reaching year 10 of tamoxifen) (RR, 0.75; 95% CI, 0.62–0.90; P = .003). The mortality reduction was also significant only after year 10 (RR, 0.71; 95% CI, 0.58–0.88; P = .0016). Extended tamoxifen use increased the risk of uterine cancer (RR, 1.74; 95% CI, 1.30–2.34; P = .0002) and pulmonary embolism (RR, 1.87; 95% CI, 1.13–3.07; P = .01), but it did not increase the risk of stroke (RR, 1.06; 95% CI, 0.83–1.36; P = .63) and was associated with decreased risk of ischemic heart disease (RR, 0.76; 95% CI, 0.60–0.95; P = .02). The cumulative risk of endometrial cancer during years 5 through 14 was 3.1% for women receiving continued tamoxifen vs 1.6% for controls (with an absolute mortality increase of 0.2%).
The aTTom study of 6,953 patients (2,755 ER-positive, 4,198 ER-untested) also reported fewer recurrences (32% vs 28%; RR, 0.85; 95% CI, 0.76–0.95; P = .003) and lower breast cancer mortality (24% vs 21%; RR, 0.88; 95% CI, 0.77–1.01; P = .05) in those allocated to 10 years of tamoxifen than in those allocated to 5 years of tamoxifen. These effects were seen in a similar time-dependent fashion, with the greatest decreases in risk of recurrence and breast cancer mortality seen after year 10. There was an increased risk of endometrial cancer (102 vs 45 endometrial cancers; RR, 2.20; 95% CI, 1.31–2.34; P < .0001), but a minimal increase in accompanying mortality related to those cancers, with 37 (1.1%) vs 20 (0.6%) deaths (absolute hazard ratio [HR], 0.5%; P = .02).
A combined analysis of the ATLAS and aTTom studies showed that it is not until year 10 of tamoxifen therapy that significant reductions in breast cancer mortality (RR, 0.75; 95% CI, 0.65–0.86; P < .05) and overall survival (RR, 0.84; 95% CI, 0.77–0.93; P < .05) are seen with longer-term tamoxifen use (10 years vs 5 years).[11] Finally, when one takes into account the reduction in breast cancer deaths seen with 5 years of tamoxifen vs no tamoxifen, 10 years of adjuvant tamoxifen use compared with no tamoxifen decreases breast cancer mortality by approximately one-third in the first 10 years of therapy, and by one-half thereafter. It is noteworthy that the ER-positive patients analyzed in the ATLAS trial combined with the patients in the aTTom trial outnumbered the patients in the NSABP B-14 extension study by twelve-fold, making these two trials the predominant source of evidence for the use of long-term tamoxifen.
A natural question is whether certain patient subsets might derive a greater degree of benefit from longer therapy. The ATLAS study showed a benefit of prolonged tamoxifen independent of age, nodal status, tumor size, previous tamoxifen duration, extent of surgery, menopausal status, previous hysterectomy, or geography. Less than 10% of the patients were premenopausal at the time of randomization (after 5 years of tamoxifen). In these premenopausal women, 24.0% of those who discontinued tamoxifen experienced disease recurrence, compared with 19.6% of those who continued tamoxifen to year 10. In comparison, in the postmenopausal women, 20.5% of those who discontinued tamoxifen experienced recurrence compared with 17.8% of those who continued tamoxifen to year 10. It is possible that continuation of tamoxifen may have benefited premenopausal patients slightly more, but statistically, menopausal status at study entry was not a significant factor in predicting continuation benefit (chi-square P value = .79). In the NSABP B-14 extension study, only one-quarter of the patients were pre/perimenopausal at the time of randomization to 5 additional years of tamoxifen or placebo, and it is not known what the outcomes of these younger patients were compared with the outcomes in the group that was postmenopausal at the time of randomization.
Because of these recent findings, major guidelines are in a state of flux. Review of existing guidelines (National Comprehensive Cancer Network [NCCN], American Society of Clinical Oncology [ASCO], St. Gallen) for hormonal therapy in pre- and postmenopausal breast cancer patients reveals some consistencies, but also some need for harmonization.[14-16] In general, the guidelines support different strategies for premenopausal and postmenopausal women. In particular, for women who are postmenopausal at the time of diagnosis, an AI should be incorporated into their adjuvant care at some point, unless a patient declines or has a contraindication to AI use. Making decisions regarding whether to prescribe AIs in an upfront, sequential, or extended manner requires attention to individual patient clinical factors and preferences, given the lack of direct comparisons of these various strategies. Further development of biomarkers of endocrine resistance and early and late recurrence will also aid future decision making in this regard.
Current Guidelines for Adjuvant Endocrine Therapy in Women Who Are Premenopausal at Diagnosis
Ovarian ablation/ovarian suppression
For women who are pre/perimenopausal at diagnosis, all three sets of guidelines concur that 5 years of tamoxifen should be recommended. They conclude that the value of ovarian ablation (OA) and ovarian suppression (OS) in addition to tamoxifen for premenopausal women is unknown; however, some of the St. Gallen panelists advocate OA/OS in women under the age of 40. All sets of guidelines are in agreement that if OS is utilized, a combination of OS and tamoxifen is preferred to OS as monotherapy or in combination with an AI.
Extended tamoxifen
The NCCN and St. Gallen guidelines state that women who are premenopausal at diagnosis and remain premenopausal after the first 5 years of tamoxifen should consider an additional 5 years of tamoxifen. For women who are premenopausal at diagnosis but who are found to be postmenopausal after completing their first 5 years of tamoxifen, options-per the NCCN guidelines-include an additional 5 years of tamoxifen, or switching to 5 years of an AI. The St. Gallen guidelines also strongly support the use of an AI after 5 years of tamoxifen therapy in node-positive postmenopausal patients, but they do not differentiate between patients who became postmenopausal while receiving tamoxifen and those who were already postmenopausal when they started tamoxifen. In contrast, the 2010 ASCO guidelines, which have not yet been updated to incorporate the recent extended-use tamoxifen trials, clearly state: “Women who are pre- or perimenopausal at diagnosis should be treated with 5 years of tamoxifen.” However, the ASCO guidelines also acknowledge that some women who were pre- or perimenopausal at the time of diagnosis may become “unequivocally postmenopausal” during tamoxifen treatment, and they recommend considering either sequential or extended AI use in this setting.
Hence, based on existing data, it is clear that extended tamoxifen should be considered for all patients who remain premenopausal after their first 5 years of tamoxifen. For patients who are premenopausal at diagnosis but who are found to be postmenopausal at the time of completion of their first 5 years of tamoxifen, treatment with an additional 5 years of tamoxifen or 5 years of an AI are both valid strategies. In the absence of clear predictors of benefit in specific patient subsets in the extended-use tamoxifen trials, the choice to prolong tamoxifen therapy or switch to an AI in appropriate patients should be based on physician and patient preferences and attention to quality of life and toxicity. Despite the failure of subset analysis to show preferential benefit for endocrine therapy in higher-risk patients, it is still recommended to take traditional baseline risk factors into account when considering extended therapy.
Existing Guidelines for Adjuvant Endocrine Therapy in Women Who Are Postmenopausal at Diagnosis
According to current guidelines, many options are available for women who are postmenopausal at diagnosis. The 2010 ASCO guidelines recommend that all postmenopausal women consider taking an AI at some point during their treatment because of the small but real (< 5%) absolute reduction in risk of recurrence with their use compared with tamoxifen; however, the ASCO guidelines acknowledge that there is no overall survival benefit for AIs over tamoxifen. They advocate treatment with AIs as either initial adjuvant therapy or after 2 to 3 years of tamoxifen, stating that “switching at 2–3 years (sequential) is recommended, but switching at 5 years is also supported by available data (extended).” The NCCN guidelines, by comparison, list as equivalent options for postmenopausal women the use of AIs as initial adjuvant therapy, as sequential therapy following 2 to 3 years of tamoxifen, and as extended therapy following 4.5 to 6 years of tamoxifen. They also state that the use of tamoxifen alone for 5 to 10 years in women who are postmenopausal at diagnosis should be reserved for those who decline or have a contraindication to AIs. In contrast, the St. Gallen panelists “strongly believed that some postmenopausal women could be treated with tamoxifen alone”-although they strongly supported starting therapy with an AI in high-risk women. All guidelines are in agreement that there is no definitive evidence at this time to support offering more than 5 years of AI therapy, although the St. Gallen panel was divided on this issue. Further, all the guidelines acknowledge that there are no data comparing an AI for 5 years, an AI for 5 years following tamoxifen for 5 years, and 10 years of tamoxifen.
Several factors drive the strong recommendation to incorporate at least some AI therapy into the adjuvant treatment of women who are postmenopausal at diagnosis. First, the majority of the sequential AI trials and all the extended adjuvant trials randomized women after completion of 2 to 5 years of tamoxifen, and thus, in effect, excluded patients with early recurrence. This represents a potentially important difference in study patient populations, compared with the primary adjuvant trials of upfront AI use vs tamoxifen. Second, compared with 5 years of tamoxifen, adjuvant AI use (primary/sequential/extended use) improves DFS and lowers the risks of breast cancer recurrence (distant and locoregional) and contralateral breast cancer.[14]
The MA.17/BIG 1-97 trial (Table 2) was a double-blind placebo-controlled trial designed to test the effectiveness of 5 years of letrozole in ER-positive breast cancer patients who had completed 4 to 6 years of adjuvant tamoxifen therapy. At the time of initial analysis for this study-at a median follow-up of 2.4 years-extended adjuvant letrozole treatment after tamoxifen had significantly reduced recurrence risk (HR, 0.57; 95% CI, 0.43–0.75; P = .00008) for patients with early-stage breast cancer.[12] This led to an early unblinding of the study, followed by an extremely high crossover rate from the placebo to the letrozole group of more than 60%. Despite this, an exploratory analysis adjusting for the treatment crossover still showed both a DFS and an overall survival benefit for letrozole compared with placebo.[17]
Unlike the extended tamoxifen trials in which no signal emerged of specific patient subgroups that would benefit from extended tamoxifen, some of the adjuvant AI trials have provided clues about patient subsets that could benefit from prolonged therapy. Interestingly, in the MA.17/BIG 1-97 trial, although all women were postmenopausal at randomization to letrozole or placebo after completion of tamoxifen, a minority of participants (877 of 4,289 patients) were premenopausal at the time of their primary diagnosis. In this small group of premenopausal women who became postmenopausal while receiving tamoxifen, the benefit of extended letrozole therapy seemed more pronounced than in the women who were postmenopausal at diagnosis.[18]
Other AI trials were prematurely closed to accrual when the initial results of the MA.17/BIG 1-97 trial were reported. One of these studies, the NSABP B-33 trial, was a double-blind trial of exemestane vs placebo as extended adjuvant therapy for postmenopausal women who were disease-free after 5 years of adjuvant tamoxifen.[19] Subset analysis of NSABP B-33 indicated that patients who were less than 60 years of age, had larger tumors, had positive lymph nodes, or had prior adjuvant chemotherapy seemed to benefit more from extended adjuvant therapy. Thus, choosing to use extended adjuvant AI dosing strategies in higher-risk patients seems prudent. Certainly this seems wise for high-risk women who start on tamoxifen at a premenopausal age and become postmenopausal during treatment. However, women who are postmenopausal at diagnosis and have high-risk features present a conundrum, because all guidelines advocate starting with a primary AI in these patients, and there are not yet data to support more than 5 years of AI therapy.
In contrast, the Austrian Breast and Colorectal Cancer Study Group (ABCSG) 6a trial (see Table 2) showed that extended anastrozole after tamoxifen reduced total recurrences (HR, 0.62; 95% CI, 0.40–0.96; P = .031) and distant recurrences (HR, 0.53; 95% CI, 0.29–0.96; P = .034).[20] Much as in the tamoxifen trials, subgroup analysis showed that the benefit seemed independent of age, nodal status, and ER positivity. However, there did seem to be a lack of benefit with extended AI therapy in the obese women who participated in this trial compared with the normal-weight women.[21]
Trials of Longer-Duration AI Therapy
At this time, there is no evidence to support prescribing more than 5 years of AI therapy. Results are eagerly awaited from a number of trials, now closed to enrollment, that are examining this issue in women with early-stage breast cancer (Table 3). The MA.17R trial randomly assigned women to treatment with letrozole or to placebo after completion of 5 years of an adjuvant AI (either as initial therapy or after tamoxifen, including in women who participated in the MA.17 study). Similarly, the NSABP B-42 trial randomized patients who had completed 5 years of hormonal therapy (either with an AI, or sequential therapy of tamoxifen followed by an AI) to 5 years of letrozole or placebo. The ABCSG-16/SALSA (Secondary Adjuvant Long-Term Study With Arimidex) study randomly assigned postmenopausal women who had had 4 to 6 years of prior endocrine therapy to extended adjuvant treatment with anastrozole for either 2 years or 5 years. The Study of Letrozole Extension (SOLE)/International Breast Cancer Study Group (IBCSG) 35-07 randomly assigned women who had completed 4 to 6 years of any prior endocrine therapy to receive 5 years of letrozole dosed either continuously or intermittently. The Different Durations of Anastrozole and Tamoxifen (DATA) trial randomly assigned women to either 5 years or 3 years of anastrozole after 5 years of any endocrine therapy. Finally, the Letrozole Adjuvant Therapy Duration (LEAD) study randomly assigned women to treatment with letrozole for either 5 years or 2 to 3 years-after 2 to 3 years of tamoxifen. In aggregate, these trials should provide guidance about longer durations of AI therapy.
Biomarkers of Late Recurrence at Diagnosis or of Hormone Resistance
The use of biomarkers that may be able to predict late recurrence at diagnosis, and the emergence of biomarkers of hormone resistance that develops over time, will hopefully assist with clinical decision making in the future. One assay that has been shown to predict late recurrence in ER-positive patients is the Breast Cancer Index (BCI). BCI is a multi-gene test that includes the two-gene ratio HOXB13:IL17BR (H/I) and the Molecular Grade Index (MGI), which was originally validated in a tamoxifen-treated cohort from Stockholm and was confirmed in a different multi-institutional cohort from the United States. A low BCI score was associated with high distant recurrence–free survival (DRFS) at 0–5 years and at > 5 years, and thus seemed to have prognostic value for late recurrence.[22] Additionally, a recent study evaluated the ability of multi-gene assays to predict early and late recurrence using archival tumor blocks from the TransATAC (translational arm of the Arimidex, Tamoxifen, Alone or in Combination trial) tissue bank for postmenopausal women with ER-positive breast cancer, in which a 21-gene recurrence score (RS) and immunohistochemical (IHC4) values had been previously ascertained. Although all of the assays were prognostic for early distant recurrence, only the BCI linear model (BCI-L) was significantly prognostic for risk of late distant recurrence (BCI-L HR, 1.95; 95% CI, 1.22–3.14; P = .0048; 21-gene RS HR, 1.13; 95% CI, 0.82–1.56; P = .47; IHC4 HR, 1.30; 95% CI, 0.88–1.94; P = .20).[23] These findings of a prognostic value of BCI score for late recurrence in samples from a large randomized study are compelling and warrant further investigations into the test’s potential for prediction of extended adjuvant treatment benefit.
Conclusions
New evidence now supports improved recurrence and breast cancer mortality outcomes with continued tamoxifen for up to 10 years in women of any age, and such long-duration therapy is especially important for women who remain premenopausal after their first 5 years of tamoxifen. If women are found to be postmenopausal after the first 5 years of tamoxifen, one can recommend an additional 5 years of tamoxifen or 5 years of an AI, depending on patient preference, compliance, and tolerance. For postmenopausal women, there is no difference in overall survival between tamoxifen and AI therapy, and the absolute reduction in recurrence is small (< 5%). It is therefore advisable to try to treat all postmenopausal patients with an AI at some point during their adjuvant therapy, with careful attention to toxicity and baseline risk. Data from trials looking at prolonged AI therapy beyond 5 years are needed to determine the optimal total duration of AI therapy. Current strategies for postmenopausal women include initial adjuvant AI therapy, sequential AI therapy after 2 to 3 years of tamoxifen, or extended AI use after 5 years of tamoxifen. Patient risk factors, compliance, toxicity, medical comorbidities, and patient preference can be considered in deciding among these strategies.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738-46.
2. Cufer T. Reducing the risk of late recurrence in hormone-responsive breast cancer. Ann Oncol. 2007;18(suppl 8):18-25.
3. Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100:1179-83.
4. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687-717.
5. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771-84.
6. Tormey DC, Gray R, Falkson HC. Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. Eastern Cooperative Oncology Group. J Natl Cancer Inst. 1996;88:1828-33.
7. Stewart HJ, Prescott RJ, Forrest AP. Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. J Natl Cancer Inst. 2001;93:456-62.
8. Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529-42.
9. Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684-90.
10. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-16.
11. Gray R. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,934 women with early breast cancer. J Clin Oncol. 2013;31(suppl):abstr 5.
12. Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793-802.
13. Petrelli F, Coinu A, Cabiddu M, et al. Five or more years of adjuvant endocrine therapy in breast cancer: a meta-analysis of published randomised trials. Breast Cancer Res Treat. 2013;140:233-40.
14. Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784-96.
15. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206-23.
16. NCCN Clinical Practice Guidelines in Oncology, Version 3.2013. Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed September 2013.
17. Jin H, Tu D, Zhao N, et al. Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover. J Clin Oncol. 2012;30:718-21.
18. Goss PE, Ingle JN, Martino S, et al. Impact of premenopausal status at breast cancer diagnosis in women entered on the placebo-controlled NCIC CTG MA17 trial of extended adjuvant letrozole. Ann Oncol. 2013;24:355-61.
19. Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008;26:1965-71.
20. Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst. 2007;99:1845-53.
21. Pfeiler G, Konigsberg R, Hadji P, et al. Impact of body mass index on estradiol depletion by aromatase inhibitors in postmenopausal women with early breast cancer. Br J Cancer. 2013;109:1522-7.
22. Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res. 2013;19:4196-205.
23. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013;14:1067-76.
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.