A Review of POEMS Syndrome
POEMS syndrome is a rare paraneoplastic syndrome that is caused by an underlying plasma cell disorder. Its main features include polyradiculoneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.
Table 1: Criteria for the Diagnosis of POEMS Syndrome
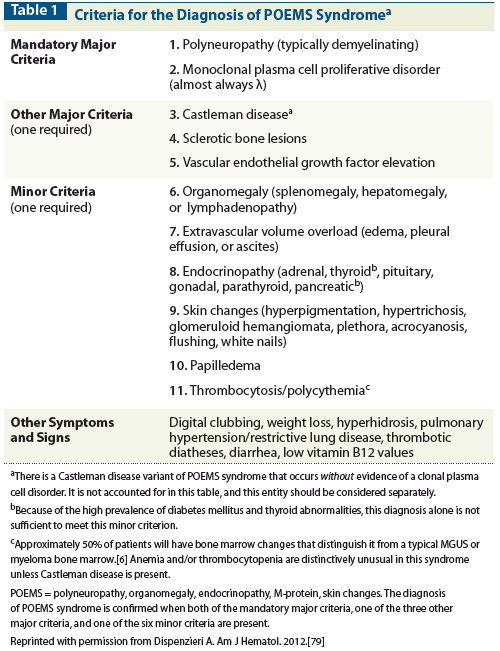
Table 2: Frequency of POEMS Syndrome Findings Based on Six Large Retrospective Series
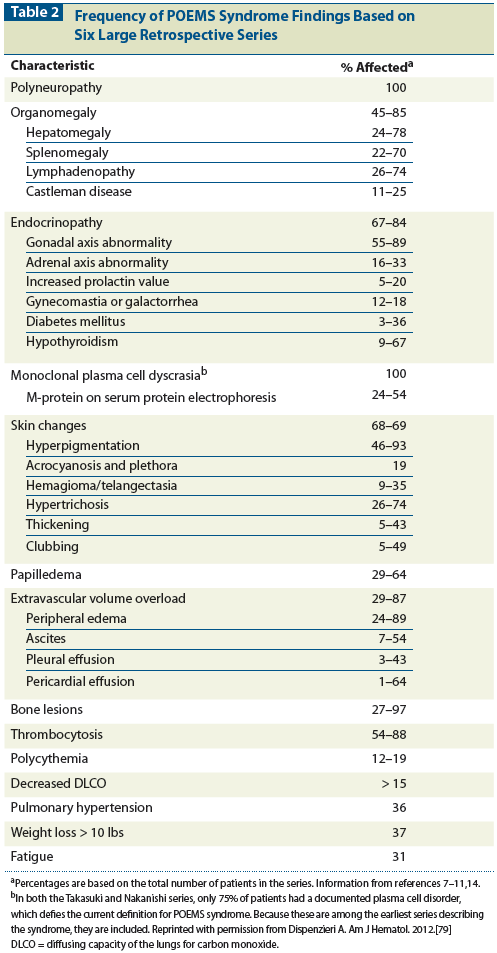
Table 3: Treatment Outcomes in Patients With POEMS Syndrome
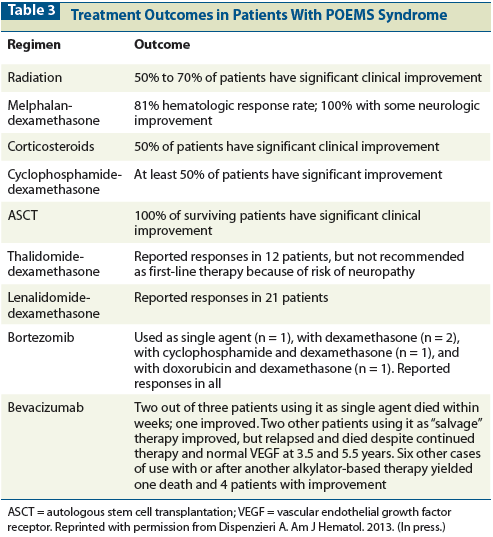
Figure 1: Classic Findings in Patients With POEMS Syndromes
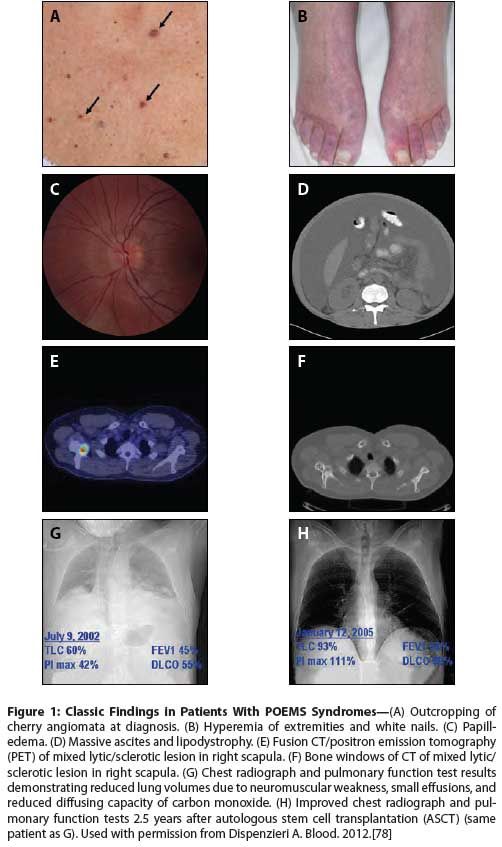
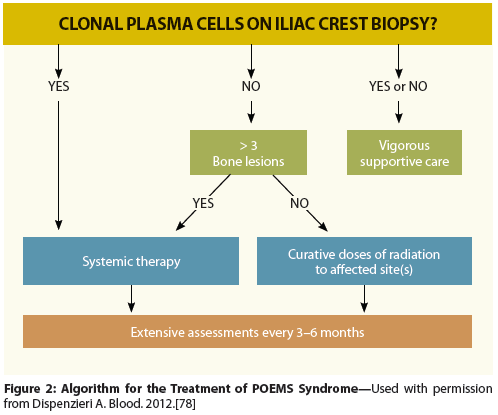
Figure 2: Algorithm for the Treatment of POEMS Syndrome
POEMS syndrome is a rare paraneoplastic syndrome that is caused by an underlying plasma cell disorder. Its main features include polyradiculoneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes. Other important characteristics of POEMS include papilledema, extravascular volume overload, sclerotic bone lesions, and thrombocytosis/ erythrocytosis (PEST). Vascular endothelial growth factor (VEGF) appears to play an important role in the disease and is especially useful for monitoring therapy, but it is not likely the sole factor driving the disease. The most commonly used therapies for POEMS include alkylators and steroids, high-dose chemotherapy with peripheral blood stem cell transplantation, lenalidomide, and bortezomib. The role of anti-VEGF antibodies is uncertain. In general, patients have an excellent prognosis if the diagnosis is made early and appropriate therapy is applied.
Introduction
POEMS syndrome, also known as Takatsuki syndrome or osteosclerotic myeloma, is a rare paraneoplastic syndrome resulting from an underlying plasma cell disorder. This acronym refers to several, but not all, of the features of the syndrome: polyradiculoneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.[1] Other important features not included in the POEMS acronym are papilledema, extravascular volume overload, sclerotic bone lesions, and thrombocytosis/erythrocytosis (PEST), as well as elevated levels of vascular endothelial growth factor (VEGF), abnormal pulmonary function tests, and a predisposition towards thrombosis. Not all features are required to make the diagnosis.
Diagnosis
The diagnosis of POEMS syndrome is made based on a composite of clinical and laboratory features (Table 1). The constellation of neuropathy and any of the following should elicit an in-depth patient evaluation for possible POEMS: a lambda-restricted monoclonal protein, thrombocytosis, anasarca, or papilledema. Aside from a good clinical history and physical examination, appropriate testing-including radiographic assessment of bones, measurement of VEGF levels,[2-5] and careful analysis of a bone marrow biopsy[6]-can differentiate this syndrome from other conditions such as chronic inflammatory polyradiculoneuropathy (CIDP), monoclonal gammopathy of undetermined significance (MGUS), neuropathy, and immunoglobulin light chain amyloid neuropathy. Table 2 illustrates the frequency of POEMS features reported in six of the largest series. The variability between series is most likely a function of retrospective reporting and promptness of diagnosis, rather than ethnic differences.[7-11]
Neuropathy is the dominant complaint. The quality and extent of the neuropathy, which is peripheral, ascending, and symmetrical, and which affects both sensation and motor function, should be elicited.[12] Pain is a dominant feature in about 10% to 15% of patients, and in one report as many as 50% of patients had hyperesthesia.[13] Autonomic neuropathy is not seen. In addition to evidence of a sensorimotor deficit, common physical findings include areflexia, a steppage gait, and a positive Romberg sign.
The presence of a monoclonal plasma cell disorder is required to make the diagnosis. This is a low “tumor” burden disease. Immunofixation of serum is most commonly required to reveal the presence of a small monoclonal protein in the blood. In about 15% of cases, however, there is no monoclonal protein detected even by immunofixation.[10] In these cases, biopsy of a sclerotic, lytic, or mixed bone lesion reveals a clone. Finally, there are rare cases in which there are no bone lesions; in such cases, blind iliac crest bone marrow biopsy often detects a small clone. Surprisingly, approximately 95% of cases are driven by a lambda light chain–restricted clone. Cases of monoclonal kappa light chains are exceedingly rare.
Organomegaly manifests commonly as hepatomegaly, splenomegaly, and/or lymphadenopathy. Castleman disease (or Castleman-like histology) is found in 11% to 30% of POEMS patients who have a documented clonal plasma cell disorder.[1,8-11] Only those with peripheral neuropathy and a plasma cell clone should be classified as having classic POEMS syndrome. Without both of these characteristics, patients can be classified as having the Castleman disease variant of POEMS if they have other POEMS features. The neuropathy in patients with Castleman disease tends to be more subtle than that of POEMS patients with osteosclerotic myeloma; it is predominantly sensory, without a motor component. In contrast to the osteosclerotic myeloma variant of POEMS, in which VEGF is the most consistently elevated cytokine, in Castleman disease interleukin 6 (IL-6) is the dominant aberrantly overexpressed cytokine. Patients with Castleman disease often have a brisk polyclonal hypergammaglobulinemia.
The endocrine features are diverse and may involve any endocrine gland function, hypothalamic function, or pituitary function. Erectile dysfunction is a common first or second symptom in male patients with POEMS. In a recent series,[14] approximately 84% of patients had a recognized endocrinopathy, with hypogonadism as the most common endocrine abnormality, followed by thyroid abnormalities, glucose metabolism abnormalities, and lastly by adrenal insufficiency. The majority of patients have evidence of multiple endocrinopathies in the four major endocrine axes (gonadal, thyroid, glucose, and adrenal).
Skin manifestations include hyperpigmentation, a recent outcropping of hemangiomas, hypertrichosis, dependent rubor and acrocyanosis, white nails, sclerodermoid changes, flushing, or nail clubbing (Figures 1A, 1B).[7,9,11,15-19] Nail clubbing is seen in ~4% of cases, but some studies have reported rates as high as 49%.[8,20]
Papilledema is present in at least one-third of patients (Figure 1C). Of the 33 patients at our institution who underwent at least one formal ophthalmologic examination during a 10-year period, about two-thirds (67%) had ocular signs and symptoms, the most common of which was papilledema, seen in 52% of those examined.[21]
Extravascular overload most commonly manifests as peripheral edema, but pleural effusion, ascites, and pericardial effusions are also common (Figure 1D). The ascites can be so severe that weekly paracentesis is required. The composition of the ascites was studied in 42 patients with POEMS syndrome. The ascitic fluid had low serum-ascites albumin gradients, consistent with an exudative process rather than a portal hypertension process in 74% of cases.[22]
Patients with POEMS syndrome are at increased risk for arterial and/or venous thromboses during the course of their disease, with nearly 20% of patients experiencing one of these complications.[23,24] Ten percent of patients present with a cerebrovascular event, most commonly embolic or vessel dissection with resulting stenosis.[25] Aberrations in the coagulation cascade have been seen in POEMS syndrome.[26]
Diagnostics
Laboratory findings are notable for an absence of cytopenias. In fact, nearly half of patients will have thrombocytosis or erythrocytosis.[10] In the series of patients with POEMS syndrome in China that was reported by Li and colleagues, 26% of the 99 patients reviewed had anemia, which the authors attributed to impaired renal function.[11] This single-institution series was enriched with Castleman disease patients (25%), a factor that may have contributed also to this unprecedented high rate of anemia.
Plasma and serum levels of VEGF are markedly elevated in POEMS[27-29] and correlate with the activity of the disease.[2,3,28] VEGF levels are independent of M-protein size.[2] The higher level observed in serum is attributable to the release of VEGF from platelets in vitro during serum processing. Other proinflammatory markers, such as interleukin 12 (IL-12), tumor necrosis factor (TNF)-alpha, and IL-6, have all been reported to be high in patients with
POEMS, but VEGF is the most consistently elevated cytokine and correlates best with disease activity.[30]
Serum creatinine levels are normal in most cases, but levels of serum cystatin C, which is a surrogate marker for renal function, are high in 71% of patients.[31] In our experience, at presentation, < 10% of patients have proteinuria exceeding 0.5 g/24 hours, and only 6% have a serum creatinine greater than or equal to 1.5 mg/dL. Four percent of patients developed renal failure as a preterminal event.[10] In a recent series from China, 37% of patients had a creatinine clearance (CrCl) of < 60 mL/min, and 9% had a CrCl < 30 mL/min. Fifteen percent had microhematuria. In our experience, renal disease is more likely to occur in patients who have co-existing Castleman disease. In POEMS syndrome, the renal histologic findings are diverse, with membranoproliferative features and evidence of endothelial injury being most common.[32] Under both light microscopy and electron microscopy, mesangial expansion, narrowing of capillary lumina, basement membrane thickening, sub-endothelial deposits, widening of the sub-endothelial space, swelling and vacuolization of endothelial cells, and mesangiolysis predominate.[33-39] Rarely, infiltration by plasma cell nests or Castleman-like lymphoma can be seen.[39]
The bone marrow biopsy reveals megakaryocyte hyperplasia and clustering in 54% and 93% of cases, respectively.[6] These megakaryocyte findings are reminiscent of a myeloproliferative disorder, but JAK2V617F mutation is uniformly absent. One-third of patients do not have clonal plasma cells on their iliac crest biopsy. These are patients who present with a solitary plasmacytoma or “multiple solitary plasmacytomas.” The other two-thirds of patients have clonal plasma cells in their bone marrow, and 91% of these cases are clonal lambda. The median percentage of plasma cells observed is less than 5%. Immunohistochemical staining is more sensitive than six-color flow cytometry in evaluating patients, because the stains provide information about bone marrow architecture, which is key in making the diagnosis in nearly half of cases. Lymphoid aggregates with clonal lambda plasma cell rimming are found in nearly half of cases.
Osteosclerotic lesions occur in approximately 95% of patients, and can be confused with benign bone islands, aneurysmal bone cysts, non-ossifying fibromas, and fibrous dysplasia.[8,10,40,41] Some lesions are densely sclerotic, whereas others are lytic with a sclerotic rim, and still others have a mixed soap-bubble appearance. Bone windows of CT body images are often very informative, often even more so than [F-18]fluorodeoxyglucose (FDG)-uptake by positron emission tomography (PET), which can be variable. Underlying these lesions are clonal plasma cells (Figures 1E, 1F).
The pulmonary manifestations are protean, including pulmonary hypertension, restrictive lung disease, impaired neuromuscular respiratory function, and impaired diffusion capacity of carbon monoxide, but many of these symptoms improve with effective therapy (Figures 1G, 1H). No direct association has been documented between the digital clubbing seen in POEMS syndrome and lung disease.
Nerve conduction studies in patients with POEMS syndrome show slowing of nerve conduction that is more predominant in the intermediate than distal nerve segments compared with CIDP, with more severe attenuation of compound muscle action potentials in the lower limbs than in the upper ones.[42-44] In contrast to CIDP, conduction block is rare.[43] The nerve biopsy shows typical features of uncompacted myelin lamellae. On ultrastructural examination there are no features of macrophage-associated demyelination, which are seen in some cases of chronic inflammatory demyelinating polyneuropathy.[45-48] However, there is evidence of endothelial cytoplasmic enlargement, opening of the tight junctions between endothelial cells, and the presence of many pinocytic vesicles adjacent to the cell membranes, which are all consistent with an alteration of the permeability of endoneurial vessels.[3] In another study of nerve biopsies in POEMS patients, more than 50% of endoneurial blood vessels had narrowed or closed lumina with thick basement membranes, strong polyclonal immunoglobulin staining in the endoneurium (consistent with opening of the blood-nerve barrier), and thrombin– anti-thrombin complexes immunohistochemically.[26]
Differential Diagnosis
The most common diagnoses in the differential for these patients are CIDP and Guillain-Barré syndrome. The best clues for differentiating POEMS syndrome these diagnoses are: (1) the lack of response to therapies that typically work in these disorders, eg, intravenous gammaglobulin or plasmapheresis; (2) the presence of other features, notably monoclonal protein, thrombocytosis, papilledema, ascites, new endocrine issues, skin changes, or sclerotic bone lesions; (3) the patient’s sense of feeling “unwell”; and (4) the presence of clonal plasma cell rimming of lymphoid aggregates found in the bone marrow.
Treatment of POEMS Syndrome
The treatment algorithm is based on the extent of the plasma cell infiltration (Figure 2). There are patients who do not have bone marrow involvement as determined by blind iliac crest sampling and others who have disseminated disease, namely diffuse bone marrow involvement and/or more than three skeletal lesions, and the approach to these two groups of patients differs. The course of POEMS syndrome is usually chronic, with a median patient survival time of nearly 14 years.[10,20] Only fingernail clubbing, extravascular volume overload (effusions, edema, and ascites),[10] and respiratory symptoms[20] have been associated with a significantly shorter overall survival time. The number of POEMS features does not affect survival.[9,24] In our experience and in a recent report by Li and colleagues, patients with co-existing Castleman disease may have an inferior overall survival compared to patients without it.[11]
In the case of patients with an isolated bone lesion without clonal plasma cells found on iliac crest biopsy, use of curative doses of radiation is the recommended therapy, akin to the management of a more straightforward solitary plasmacytoma of bone. Not only does radiation to an isolated lesion (or even several isolated lesions) improve the symptoms of POEMS syndrome, but such treatment also may be curative, similar to the cures achieved in patients with solitary plasmacytoma of bone. In a series of 35 patients with POEMS syndrome treated at the Mayo Clinic, Rochester, Minnesota, radiation was used as primary therapy.[49] This resulted in a 4-year overall survival rate of 97% and a 4-year failure-free survival rate of 52%. More than half of the “failures” occurred within 12 months of radiation. It is unclear in this retrospective series whether these were true failures or were driven by patient and physician anxiety over slow response to treatment.
Once there is disseminated bone marrow involvement, albeit even in patients with a low plasma cell percentage, radiation is not expected to be curative. If the bone lesion (ie, plasmacytoma) is reasonably large, then radiation can be considered as primary therapy despite a positive iliac crest biopsy. One approach in this type of case is to follow patients’ symptoms and levels of serum M-protein and blood VEGF level over the course of 6 to 12 months after they have completed radiation therapy, and then decide whether systemic treatment should be added. More commonly, however, once disseminated disease has been identified, systemic therapy is recommended with the caveat that large bony lesions with a significant lytic component may require adjuvant radiation therapy. Decisions about adjuvant radiation should be made on a case-by-case basis and typically not until a minimum of 6 to 12 months after completion of chemotherapy. It is imperative that the treating physician (and patient) realize that there is a lag between completion of successful therapy and neurologic response, often with no discernible improvement until 3 to 6 months after completion of therapy. Maximal response is not seen until 2 to 3 years afterwards. Other features, such as anasarca, papilledema, and even skin changes, typically improve sooner. Optimal FDG-PET response may also lag by 6 to 12 months.
Because no randomized clinical trials have been conducted in patients with POEMS syndrome, treatment recommendations are based on case series and anecdote. Despite the relationship between disease response and dropping levels of VEGF, most of the successful outcomes in POEMS have been associated with therapy directed at the underlying clonal plasma cell disorder rather than with antibody treatment that solely targets VEGF. Table 3 summarizes observed outcomes. Corticosteroids may provide symptomatic improvement, but response duration is limited. The most extensive experience has been with alkylator-based therapy, either low-dose or high-dose with peripheral blood stem cell transplant. The first prospective clinical trial to treat POEMS syndrome included 31 patients who were treated with 12 cycles of melphalan and dexamethasone.[50] Eighty-one percent of patients had hematologic response, 100% had VEGF response, and 100% had at least some improvement in neurologic status. A limitation of this study is that follow-up is only 21 months, so long-term outcomes are not yet available. Personal experiences and retrospective reports of the use of cyclophosphamide-based therapy are also promising.
High-dose chemotherapy with peripheral blood stem cell transplant can also be quite effective, but selection bias may confound reports of this approach. Case series, which include approximately 100 patients altogether, suggest that 100% of patients achieve at least some neurologic improvement.[51] Anecdotally, responses are durable, but relapses have been reported.[52,53] Seventy-five percent of patients are progression-free at 5 years.[54] Doses of melphalan ranging from 140 mg/m2 to 200 mg/m2 are used, with the lower doses used for sicker patients. In addition, tandem transplant has been employed in one patient, but again, no information is available regarding any added value of the second transplant.[55] Treatment-related morbidity and mortality can be minimized by recognizing and treating an engraftment-type syndrome that is characterized by fevers, rash, diarrhea, weight gain, and respiratory symptoms and signs, and which occurs between days 7 and 15 post stem cell infusion.[56] A starting dose of prednisone of between 20 mg/day and 1,500 mg/day has been used. No evidence-based recommendation can be made as to the appropriate dose, but our personal experience would place the daily starting dose anywhere between 1–2 mg/kg and 500 mg. The taper can typically start within 2 days, and should be completed no sooner than 10 days after initiation of treatment. Splenomegaly may predict a complicated peri-transplant course. POEMS patients have a higher-than-expected transfusion need compared with myeloma patients.
Other promising treatments include lenalidomide, thalidomide, and bortezomib, all of which can have anti-VEGF and anti-TNF effects. Lenalidomide with dexamethasone appears to be quite effective, but relapses have been reported.[57-59] In the French series of 20 patients, all of whom responded, 4 relapsed 3 to 10 months after the end of treatment; however, 3 of these patients in whom treatment failed responded to further therapy, including one who responded to re-introduction of the lenalidomide-dexamethasone combination.[59] Given the intrinsic risk of thrombosis in patients with POEMS syndrome, it is imperative that at least an aspirin be used for prophylaxis. The bleeding risk associated with use of low-molecular-weight heparin or warfarin should be balanced against the risk of falls in POEMS syndrome patients.
Bortezomib use has been reported in five patients.[60-64] The first report is difficult to interpret because the patient had been treated with several chemotherapy drugs prior to receiving combination therapy with bortezomib, doxorubicin, and dexamethasone. There was early evidence of improvement even before the patient started the bortezomib regimen. The other reports, which describe outcomes of bortezomib given as a single agent, with dexamethasone, or with dexamethasone and cyclophosphamide all showed remarkable improvements in POEMS syndrome patients, without any worsening of the peripheral neuropathy. Dramatic improvement in ascites was seen in more than one instance.
Thalidomide in combination with dexamethasone has also been shown to produce responses in terms of VEGF inhibition, peripheral neuropathy, and extravascular volume overload, but hematologic responses have not been reported.[65-68] Enthusiasm for this therapy should be tempered by the risk of peripheral neuropathy associated with this drug.
Although an anti-VEGF strategy is appealing, results with bevacizumab have been mixed.[51,52,69-73] Three patients who had also received alkylator therapy during and/or preceding bevacizumab therapy had benefit,[69,72,73] including one patient who improved but was then consolidated with high-dose chemotherapy with autologous stem cell transplantation.[73] Three patients who received bevacizumab died.[52,70,74] Both our experience and the medical literature would support that single-agent intravenous immunoglobulin (IV IG) or plasmapheresis is not helpful. A recent report, however, described reduction in serum VEGF and clinical improvement with single-agent IV IG. The response was not durable, which prompted another course of IV IG, with radiation to a solitary plasmacytoma.[75] Other treatments, such as interferon-alpha, tamoxifen, trans-retinoic acid, ticlopidine, argatroban, and strontium-89, have been reported to have activity, mostly in single-patient case reports.[24]
Attention to supportive care is imperative. Orthotics, physical therapy, and continuous positive airway pressure (CPAP) all play important roles in patient recovery. Ankle foot orthotics can increase mobility and reduce falls. Physical therapy reduces the risk of permanent contractures and leads to improved function, over both the long and short term. For those with severe neuromuscular weakness, CPAP and/or bi-level positive airway pressure (biPAP) provides better oxygenation and potentially reduces the risk of complications associated with hypoventilation, such as pulmonary infection and pulmonary hypertension.
Patients must be followed carefully on a quarterly basis, by tracking the status of deficits and comparing these to baseline. VEGF responses may occur as soon as 3 months after initiation of therapy,[76] but they can be delayed. VEGF is an imperfect marker in that discordance between disease activity and response have been reported,[77] so trends rather than absolute values should direct therapeutic decisions. Serum M-protein responses as measured by protein electrophoresis, immunofixation electrophoresis, or serum immunoglobulin free light chains also pose a challenge. The size of the M-protein is typically small, making standard multiple myeloma response criteria inapplicable in most cases. In addition, patients can derive significant clinical benefit from therapy in the absence of an M-protein response.[50,56] Finally, despite the fact that levels of immunoglobulin free light chains are elevated in 90% of POEMS patients, the ratio is normal in all but 18%,[31] making the ratio of limited value for patients with POEMS syndrome.
Financial Disclosure: Dr. Dispenzieri receives research support from Celgene, Millennium, Pfizer, and Janssen. Dr. Buadi has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Acknowledgment:Dr. Dispenzieri and this work are supported in part by National Institutes of Health grants CA168719 and CA107476, and by the Andrew and Lillian A. Posey Foundation, the Predolin Foundation, and the Jabbs Foundation.
References:
1. Bardwick PA, Zvaifler NJ, Gill GN, et al. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine. 1980;59:311-22.
2. Watanabe O, Maruyama I, Arimura K, et al. Overproduction of vascular endothelial growth factor/vascular permeability factor is causative in Crow-Fukase (POEMS) syndrome. Muscle Nerve. 1998;21:1390-7.
3. Scarlato M, Previtali SC, Carpo M, et al. Polyneuropathy in POEMS syndrome: role of angiogenic factors in the pathogenesis. Brain. 2005;128:1911-20.
4. Nobile-Orazio E, Terenghi F, Giannotta C, et al. Serum VEGF levels in POEMS syndrome and in immune-mediated neuropathies. Neurology. 2009;72:1024-6.
5. Briani C, Fabrizi GM, Ruggero S, et al. Vascular endothelial growth factor helps differentiate neuropathies in rare plasma cell dyscrasias. Muscle Nerve. 2010;43:164-7.
6. Dao LN, Hanson CA, Dispenzieri A, et al. Bone marrow histopathology in POEMS syndrome: a distinctive combination of plasma cell, lymphoid and myeloid findings in 87 patients. Blood. 2011;117:6438-44.
7. Takatsuki K, Sanada I. Plasma cell dyscrasia with polyneuropathy and endocrine disorder: clinical and laboratory features of 109 reported cases. Jpn J Clin Oncol. 1983;13:543-55.
8. Nakanishi T, Sobue I, Toyokura Y, et al. The Crow-Fukase syndrome: a study of 102 cases in Japan. Neurology. 1984;34:712-20.
9. Soubrier MJ, Dubost JJ, Sauvezie BJ. POEMS syndrome: a study of 25 cases and a review of the literature. French Study Group on POEMS Syndrome. Am J Med. 1994;97:543-53.
10. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101:2496-506.
11. Li J, Zhou DB, Huang Z, et al. Clinical characteristics and long-term outcome of patients with POEMS syndrome in China. Ann Hematol. 2011;90:819-26.
12. Kelly JJ, Jr, Kyle RA, Miles JM, Dyck PJ. Osteosclerotic myeloma and peripheral neuropathy. Neurology. 1983;33:202-10.
13. Koike H, Iijima M, Mori K, et al. Neuropathic pain correlates with myelinated fibre loss and cytokine profile in POEMS syndrome. J Neurol Neurosurg Psychiatry. 2008;79:1171-9.
14. Ghandi GY, Basu R, Dispenzieri A, et al. Endocrinopathy in POEMS syndrome: the Mayo Clinic experience. Mayo Clin Proc. 2007;82:836-42.
15. Singh D, Wadhwa J, Kumar L, et al. POEMS syndrome: experience with fourteen cases. Leuk Lymphoma. 2003;44:1749-52.
16. Zhang B, Song X, Liang B, et al. The clinical study of POEMS syndrome in China. Neuro Endocrinol Lett. 2010;31:229-37.
17. Kulkarni GB, Mahadevan A, Taly AB, et al. Clinicopathological profile of polyneuropathy, organomegaly, endocrinopathy, M protein and skin changes (POEMS) syndrome. J Clin Neurosci. 2011;18:356-60.
18. Barete S, Mouawad R, Choquet S, et al. Skin manifestations and vascular endothelial growth factor levels in POEMS syndrome: impact of autologous hematopoietic stem cell transplantation. Arch Dermatol. 2010;146:615-23.
19. Caramaschi P, Biasi D, Lestani M, Chilosi M. A case of acquired partial lipodystrophy associated with POEMS syndrome. Rheumatology. 2003;42:488-90.
20. Allam JS, Kennedy CC, Aksamit TR, Dispenzieri A. Pulmonary manifestations in patients with POEMS syndrome: a retrospective review of 137 patients. Chest. 2008;133:969-74.
21. Kaushik M, Pulido JS, Abreu R, et al. Ocular findings in patients with polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes syndrome. Ophthalmology. 2011;118:778-82.
22. Cui RT, Yu SY, Huang XS, et al. The characteristics of ascites in patients with POEMS syndrome. Ann Hematol 2013;92:1661-4.
23. Lesprit P, Authier FJ, Gherardi R, et al. Acute arterial obliteration: a new feature of the POEMS syndrome? Medicine. 1996;75:226-32.
24. Dispenzieri A. POEMS syndrome. Blood Rev. 2007;21:285-99.
25. Dupont SA, Dispenzieri A, Mauermann ML, et al. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73:1308-12.
26. Saida K, Kawakami H, Ohta M, Iwamura K. Coagulation and vascular abnormalities in Crow-Fukase syndrome. Muscle Nerve. 1997;20:486-92.
27. Watanabe O, Arimura K, Kitajima I, et al. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome [letter]. Lancet. 1996;347:702.
28. Soubrier M, Dubost JJ, Serre AF, et al. Growth factors in POEMS syndrome: evidence for a marked increase in circulating vascular endothelial growth factor. Arthritis Rheum. 1997;40:786-7.
29. Hashiguchi T, Arimura K, Matsumuro K, et al. Highly concentrated vascular endothelial growth factor in platelets in Crow-Fukase syndrome. Muscle Nerve. 2000;23:1051-6.
30. D’Souza A, Hayman SR, Buadi F, et al. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood. 2011;118:4663-5.
31. Stankowski-Drengler T, Gertz MA, Katzmann JA, et al. Serum immunoglobulin free light chain measurements and heavy chain isotype usage provide insight into disease biology in patients with POEMS syndrome. Am J Hematol. 2010;85:431-4.
32. Sanada S, Ookawara S, Karube H, et al. Marked recovery of severe renal lesions in POEMS syndrome with high-dose melphalan therapy supported by autologous blood stem cell transplantation. Am J Kidney Dis. 2006;47:672-9.
33. Navis GJ, Dullaart RP, Vellenga E, et al. Renal disease in POEMS syndrome: report on a case and review of the literature. Nephrol Dial Transplant. 1994;9:1477-81.
34. Viard JP, Lesavre P, Boitard C, et al. POEMS syndrome presenting as systemic sclerosis. Clinical and pathologic study of a case with microangiopathic glomerular lesions. Am J Med. 1988;84:524-8.
35. Sano M, Terasaki T, Koyama A, et al. Glomerular lesions associated with the Crow-Fukase syndrome. Virchows Arch A, Pathol Anat Histopathol. 1986;409:3-9.
36. Takazoe K, Shimada T, Kawamura T, et al. Possible mechanism of progressive renal failure in Crow-Fukase syndrome [letter]. Clin Nephrol. 1997;47:66-7.
37. Mizuiri S, Mitsuo K, Sakai K, et al. Renal involvement in POEMS syndrome. Nephron. 1991;59:153-6.
38. Stewart PM, McIntyre MA Edwards CR. The endocrinopathy of POEMS syndrome. Scott Med J. 1989;34:520-2.
39. Nakamoto Y, Imai H, Yasuda T, et al. A spectrum of clinicopathological features of nephropathy associated with POEMS syndrome. Nephrol Dial Transplant. 1999;14:2370-8.
40. Tanaka O, Ohsawa T. The POEMS syndrome: report of three cases with radiographic abnormalities. Radiologe. 1984;24:472-4.
41. Chong ST, Beasley HS, Daffner RH. POEMS syndrome: radiographic appearance with MRI correlation. Skeletal Radiol. 2006;35:690-5.
42. Kelly JJ, Jr. The electrodiagnostic findings in peripheral neuropathy associated with monoclonal gammopathy. Muscle Nerve. 1983;6:504-9.
43. Sung JY, Kuwabara S, Ogawara K, et al. Patterns of nerve conduction abnormalities in POEMS syndrome. Muscle Nerve. 2002;26:189-93.
44. Min JH, Hong YH, Lee KW. Electrophysiological features of patients with POEMS syndrome. Clin Neurophysiol. 2005;116:965-8.
45. Vital C, Vital A, Ferrer X, et al. Crow-Fukase (POEMS) syndrome: a study of peripheral nerve biopsy in five new cases. J Peripher Nerv Syst. 2003;8:136-44.
46. Orefice G, Morra VB, De Michele G, et al. POEMS syndrome: clinical, pathological and immunological study of a case. Neurol Res. 1994;16:477-80.
47. Crisci C, Barbieri F, Parente D, et al. POEMS syndrome: follow-up study of a case. Clin Neurol Neurosurg. 1992;94:65-8.
48. Bergouignan FX, Massonnat R, Vital C, et al. Uncompacted lamellae in three patients with POEMS syndrome. Eur Neurol. 1987;27:173-81
49. Humeniuk MS, Gertz MA, Lacy MQ, et al. Outcomes of patients with POEMS syndrome treated initially with radiation. Blood. 2013;122:68-73
50. Li J, Zhang W, Jiao L, et al. Combination of melphalan and dexamethasone for patients with newly diagnosed POEMS syndrome. Blood. 2011;117:6445-9.
51. Dispenzieri A. POEMS syndrome: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:591-601.
52. Samaras P, Bauer S, Stenner-Liewen F, et al. Treatment of POEMS syndrome with bevacizumab. Haematologica. 2007;92:1438-9.
53. Imai N, Taguchi J, Yagi N, et al. Relapse of polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes (POEMS) syndrome without increased level of vascular endothelial growth factor following successful autologous peripheral blood stem cell transplantation. Neuromuscul Disord. 2009;19:363-5.
54. D’Souza A, Lacy M, Gertz M, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood. 2012;120:56-62.
55. Kojima H, Katsuoka Y, Katsura Y, et al. Successful treatment of a patient with POEMS syndrome by tandem high-dose chemotherapy with autologous CD34+ purged stem cell rescue. Int J Hematol. 2006;84:182-5.
56. Dispenzieri A, Lacy MQ, Hayman SR, et al. Peripheral blood stem cell transplant for POEMS syndrome is associated with high rates of engraftment syndrome. Eur J Haematol. 2008;80:397-406.
57. Vannata B, Laurenti L, Chiusolo P, et al. Efficacy of lenalidomide plus dexamethasone for POEMS syndrome relapsed after autologous peripheral stem-cell transplantation. Am J Hematol. 2012;87:641-2.
58. Dispenzieri A, Klein CJ, Mauermann ML. Lenalidomide therapy in a patient with POEMS Syndrome. Blood. 2007;110:1075-6.
59. Royer B, Merlusca L, Abraham J, et al. Efficacy of lenalidomide in POEMS syndrome: A retrospective study of 20 patients. Am J Hematol. 2013;88:207-12.
60. Tang X, Shi X, Sun A, et al. Successful bortezomib-based treatment in POEMS syndrome. Eur J Haematol. 2009;83:609-10.
61. Kaygusuz I, Tezcan H, Cetiner M, et al. Bortezomib: a new therapeutic option for POEMS syndrome. Eur J Haematol. 2010;84:175-7.
62. Zeng K, Yang JR, Li J, et al. Effective induction therapy with subcutaneous administration of bortezomib for newly diagnosed POEMS syndrome: a case report and a review of the literature. Acta Haematologica. 2013;129:101-5.
63. Warsame R, Kohut IE, Dispenzieri A. Successful use of cyclophosphamide, bortezomib, and dexamethasone to treat a case of relapsed POEMS. Eur J Haematol. 2012;88:549-50.
64. Ohguchi H, Ohba R, Onishi Y, et al. Successful treatment with bortezomib and thalidomide for POEMS syndrome. Ann Hematol. 2011;90:1113-4.
65. Inoue D, Kato A, Tabata S, et al. Successful treatment of POEMS syndrome complicated by severe congestive heart failure with thalidomide. Intern Med. 2010;49:461-6.
66. Kuwabara S, Misawa S, Kanai K, et al. Thalidomide reduces serum VEGF levels and improves peripheral neuropathy in POEMS syndrome. J Neurol Neurosurg Psychiatry. 2008;79:1255-7.
67. Kim SY, Lee SA, Ryoo HM, et al. Thalidomide for POEMS syndrome. Ann Hematol. 2006;85:545-6.
68. Sinisalo M, Hietaharju A, Sauranen J, Wirta O. Thalidomide in POEMS syndrome: case report. Am J Hematol. 2004;76:66-8.
69. Badros A, Porter N, Zimrin A. Bevacizumab therapy for POEMS syndrome. Blood. 2005;106:1135.
70. Straume O, Bergheim J, Ernst P. Bevacizumab therapy for POEMS syndrome. Blood. 2006;107:4972-3.
71. Dietrich PY, Duchosal MA. Bevacizumab therapy before autologous stem-cell transplantation for POEMS syndrome. Ann Oncol. 2008;19:595.
72. Badros A. Bevacizumab therapy for POEMS syndrome. Blood. 2006;107.
73. Ohwada C, Nakaseko C, Sakai S, et al. Successful combination treatment with bevacizumab, thalidomide and autologous PBSC for severe POEMS syndrome. Bone Marrow Transplant. 2009;43:739-40.
74. Kanai K, Kuwabara S, Misawa S, Hattori T. Failure of treatment with anti-VEGF monoclonal antibody for long-standing POEMS syndrome. Intern Med. 2007; 46:311-3.
75. Terracciano C, Fiore S, Doldo E, et al. Inverse correlation between VEGF and soluble VEGF receptor 2 in POEMS with AIDP responsive to intravenous immunoglobulin. Muscle Nerve. 2010;42:445-8.
76. Kuwabara S, Misawa S, Kanai K, et al. Neurologic improvement after peripheral blood stem cell transplantation in POEMS syndrome. Neurology. 2008; 71:1691-5.
77. Goto H, Nishio M, Kumano K, et al. Discrepancy between disease activity and levels of vascular endothelial growth factor in a patient with POEMS syndrome successfully treated with autologous stem-cell transplantation. Bone Marrow Transplant. 2008; 42:627-9.
78. Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119:5650-8.
79. Dispenzieri A. POEMS syndrome: update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87:804-14.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.