Adult Burkitt Lymphoma: Advances in Diagnosis and Treatment
Burkitt lymphoma (BL) is a unique B-cell lymphoma characterized by a high proliferation rate and cytogenetic changes related to c-myc proto-oncogene overexpression. Burkitt lymphoma is a highly aggressive B-cell lymphoma that is most frequently seen in children and young adults in endemic areas.
ABSTRACT: Burkitt lymphoma is a unique B-cell malignancy with a high proliferation rate and characteristic genetic changes involving the c-myc oncogene. Burkitt lymphoma is common in children but also occurs in adults, where distinction from diffuse large B-cell lymphoma may pose a problem. The development of brief, very intensive chemotherapy regimens has led to a very high cure rate in children with Burkitt lymphoma. The use of these regimens in adults, often in combination with the antibody rituximab (Rituxan), has also made the cure of a majority of adults possible. Burkitt lymphoma in adults cannot be treated effectively with the common regimens used for diffuse large B-cell lymphoma such as CHOP-R (cyclophosphamide, doxorubicin HCl, vincristine [Oncovin], prednisone, rituximab). Prompt diagnosis and initiation of appropriate therapy with attention to the possibility of tumor lysis syndrome are necessary for optimal results.
Burkitt lymphoma (BL) is a unique B-cell lymphoma characterized by a high proliferation rate and cytogenetic changes related to c-myc proto-oncogene overexpression. Burkitt lymphoma is a highly aggressive B-cell lymphoma that is most frequently seen in children and young adults in endemic areas. As a result of the worldwide epidemic of acquired immunodeficiency syndrome (AIDS), the number of cases of adult BL has increased substantially in the past 3 decades.
TABLE 1
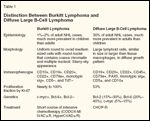
Distinction Between Burkitt Lymphoma and Diffuse B-Cell Lymphoma
The challenge of correctly distinguishing BL in an individual patient becomes particularly critical when the clinician is confronted with a treatment decision. Burkitt lymphoma is rapidly fatal if not treated appropriately with brief intensive chemotherapy, which has yielded an excellent result. However, when patients with BL are treated with regimens for diffuse large B-cell lymphoma (DLBCL), the outcome is usually poor.[1-4] On the other hand, brief intensive therapy is not the favored choice for DLBCL since it is associated with significant adverse effects (Table 1). Therefore, use of more advanced modalities to establish an accurate diagnosis is crucial, to avoid undertreatment or overtreatment of patients. The aim of this review is to discuss adult BL and emphasize controversial topics in diagnosis and treatment.
Historical Background and Epidemiology
Burkitt lymphoma was first described by Dr. Dennis Burkitt, an Irish surgeon who was working for the Colonial Medical Service in Uganda. He noted a high incidence of rapid-growing tumors affecting the jaws of African children in the endemic areas of malaria. At that time, Dr. Burkitt described the tumor as a form of sarcoma.[5] Three years later, the tumor was recognized histologically as a malignant lymphoma by Burkitt and O’Conor when they studied a series of cases-involving extranodal sites in African children-that shared the geographic distribution, histologic features, and high incidence of jaw involvement.[6]
Burkitt lymphoma accounts for over half of all childhood cancers in endemic areas, and 40% to 50% of childhood non-Hodgkin lymphomas (NHLs) in nonendemic areas (America and Western Europe).[7] BL is a rare lymphoma in adults, except in human immunodeficiency virus (HIV)-positive patients. It constitutes 1% to 2% of all non-HIV adult lymphomas in Western Europe and the United States.[8]
TABLE 2

Burkitt Lymphoma Variants
The World Health Organization (WHO) classified BL on the basis of geographic distribution and clinical presentation into three subtypes: endemic, sporadic, and immunodeficiency-associated BL (Table 2). These subtypes share the same morphologic and immunohistologic features.
Endemic BL
The endemic variant is the prototype and the most common form. It describes cases that have been observed in children in equatorial Africa and Papua New Guinea, with a geographic distribution pattern that corresponds with endemic falciparum malaria. The highest risk of BL is seen between 10° north and south of the equator.[9] The disease has a tendency to occur in low, warm, and humid lands,[10] and it is an important health issue in areas such as the Middle East, North Africa, and parts of South America. It is characterized by a high incidence of jaw and other facial bone involvement. Central nervous system (CNS) and bone marrow involvement in children has been reported in 12% and 22% of cases, respectively.[11] Nearly all endemic cases are associated with EBV infection.[12]
Sporadic BL
In contrast, the sporadic form describes cases that occur outside the endemic distribution of the disease and is seen in industrialized nations such as North America and Europe. It accounts for a minor percentage of adult lymphomas, and its peak incidence occurs in the second and third decades.
Sporadic BL is a highly aggressive disease with a propensity to invade bone marrow and CNS, with a reported incidence of 30% to 38% and 13% to 17% of cases, respectively.[13] Lymph node involvement is more common among adults than children.[14] Whereas the jaw is the most common affected site in the endemic form, it is infrequently involved in sporadic Burkitt.[15] The abdomen is the most common site in sporadic cases, particularly the terminal ileum, cecum, and intra-abdominal lymph nodes. However, it also occurs in sites such as the ovary, kidney, pancreas, liver, omentum, Waldeyer’s ring, and breast.[16] Breast involvement is observed almost exclusively in girls at the onset of puberty and in lactating women.[17]
One-third of patients have B symptoms at presentation (unexplained fever higher than 38°C (100.4°F) in the prior month, unexplained weight loss greater than 10% in the past 6 months, and recurrent drenching night sweats in the prior month). Patient with abdominal disease usually present with abdominal mass or pain, bowel obstruction, gastrointestinal bleeding, or a syndrome mimicking appendicitis. In the sporadic form, 15% to 30% neoplastic cells are EBV-positive.[18]
Immunodeficiency-Associated BL
TABLE 3

St. Jude/Murphy Staging System for Burkitt Lymphoma
The third group is immunodeficiency-associated BL which is seen in HIV-positive patients, to a lesser extent in posttransplant recipients,[19,20] and in some individuals with congenital immunodeficiency.[21] Burkitt lymphoma occurs over a thousand times more often in HIV-positive individuals than in the general population.[22] It accounts for 30% to 40% of all HIV-associated NHLs.[23] Compared to other AIDS-associated NHLs, BL occurs in younger patients with higher CD4 counts. Since the introduction of highly active antiretroviral therapy (HAART), the prognosis of HIV-associated NHL has improved significantly with standard chemotherapy protocols, except for BL, which continues to carry an unsatisfied prognosis with current chemotherapy.[24]
The majority of patients are diagnosed with stage III or IV disease (Table 3).[25] Relapse with CNS involvement tends to occur early in the course of the disease.[26] Posttransplant BL tends to occur after a relatively long interval following the transplant (average interval was 4.5 years in one series).[19]
Pathology and Pathobiology
TABLE 4

Pathologic Variants of Burkitt Lymphoma
Histologically, classic BL is characterized by a uniform proliferation of medium-sized cells with round nuclei, stippled chromatin, and multiple small, membrane-associated nucleoli (Table 4). The cells have a moderate amount of basophilic cytoplasm, with numerous lipid vacuoles in smears and touch preparations. The hallmark of BL is the presence of a “starry sky” appearance seen at low-power magnification. This appearance is created by numerous macrophages containing ingested fragments of tumor cells as a consequence of rapid proliferation and a high rate of apoptosis (Figure 1). The rate of cell division is extremely high, as reflected by the presence of numerous mitotic figures and a high fraction of proliferating cells (> 95%) as demonstrated by Ki-67 stains. This classic form of BL is seen in most endemic cases and in most sporadic pediatric cases.[2]
FIGURE 1
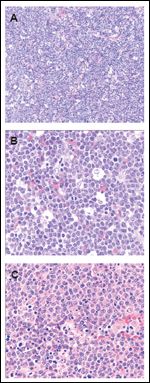
Morphologic Features of Burkitt Lymphoma
The WHO classification has identified Burkitt lymphoma and leukemia as a single entity of mature B-cell lymphoma with two related morphologic variants in addition to the classic form: BL with plasmacytoid differentiation and atypical BL/Burkitt-like lymphoma. These variants share the genetic and immunophenotypic features of classic BL, but they have atypical morphologic features.[27] Atypical Burkitt lymphoma (ABL) has morphologic features intermediate between BL and DLBCL,[3] with greater pleomorphism in nuclear size and shape, cells with more prominent central nucleoli, and the presence of large centroblasts admixed, and it is seen more frequently in sporadic adult cases (Figure 1). The revised European-American lymphoma (REAL) classification gave ABL a provisional status,[28] which was a confusing entity for clinicians, who often found it difficult to choose between treating the disease as BL or as DLBCL. The Southwest Oncology Group reported that ABL is an entity of high-grade lymphoma much closer to BL than DLBCL, which can be differentiated by its characteristic phenotypic and molecular features, and a higher proliferation index than is usually seen in DLBCL.[29] The WHO classification resolved this dilemma by recognizing ABL as a morphologic variant of BL that requires intensive therapy.
BL with plasmacytoid differentiation is seen frequently in AIDS patients. It is distinguished by features of ABL and the presence of monotypic cytoplasmic immunoglobulin.
The features of BL are consistent with a germinal center-cell stage of differentiation, based on the presence of somatically mutated immunoglobulin heavy chain variable-region genes and the expression of characteristic GC B-cell surface markers such as CD10 and Bcl-6.[30-32] Some cases of endemic BL show evidence of ongoing somatic hypermutation, which supports its germinal center-cell origin.[33]
Immunophenotypic Features
The cells of BL typically express monotypic surface IgM, CD19, CD20, CD22, CD10, Bcl-6, and CD79a, and are negative for CD5, CD23, Bcl-2, and nuclear terminal deoxyribonucleotide transferase (TdT).[8,34] Lack of surface immunoglobulin has been reported in a few cases.[18] The presence of CD10 and Bcl-6 expression supports the germinal center-cell stage of differentiation.[35] A remarkable feature of BL is the high growth fraction (> 95%) as demonstrated by Ki-67. The leukemic cells of BL express a mature immunophenotype that distinguishes it from precursor B-cell acute lymphoblastic leukemia (ALL).[36]
Atypical BL demonstrates more phenotypic diversity and may exhibit a lower proliferation rate compared to the classic form and more frequent expression of Bcl-2.[37,38] On the other hand, some cases of DLBCL exhibit an overlapping immunophenotype with BL including a high proliferation rate,[39] which makes the distinction of ABL from DLBCL difficult based on immunophenotypic characteristics alone. Expression of CD21-the EBV receptor-is seen in EBV-positive cases.[40] It is present in the vast majority of endemic cases of BL.
Molecular Genetics
TABLE 5

Treatment of Burkitt Lymphoma
C-myc encodes a transcriptional factor. It has a marked influence on cell proliferation, growth, cellular differentiation, and apoptosis.[41] Dysregulation of the gene is the key element in BL pathogenesis at the molecular level.
Translocation of the c-myc oncogene is universal in BL. This abnormality juxtaposes the c-myc oncogene location at 8q24 and one of three immunoglobulin loci. The most common translocation is (8;14), which is seen in 80% of BL cases; it occurs between the c-myc oncogene and Ig heavy chain gene (IgH). About 15% of BL cases have a t(2;8) rearrangement, where the translocation occurs between c-myc and kappa light chain gene, and the remaining 5% have an (8;22) translocation between c-myc and lambda light chain gene.[42] The detection of translocations is not always feasible by performing a routine cytogenetic assessment. Fluorescence in situ hybridization (FISH) using a break-apart probe or long-segment polymerase chain reaction increases the chance of identifying the presence of these translocations.[18]
The position of the breakpoint in relation to c-myc gene at chromosome 8 and IgH at chromosome 14 is not variable. Studies have found a correlation between the site of the breakpoint and the geographic distribution of BL. In the endemic form, the breakpoint on chromosome 8 tends to occur upstream of the c-myc gene, while the breakpoint in the IgH locus is usually located within the joining segment. In sporadic as well as AIDS-associated cases, the translocation breakpoint often falls within the c-myc gene on chromosome 8 and in the IgH switch region in chromosome 14.[41,43-45] This may imply a diverse pathogenesis for the variants of BL, which may explain the clinical variations between the endemic and sporadic subtypes.
Making BL diagnosis more challenging, c-myc was found to be sometimes overexpressed in DLBCL, as 5% to 15% of DLBCL cases harbor this rearrangement.[34,46] Considering that 40% of NHLs are DLBCL and 10% of these cases involve c-myc translocation, in contrast to BL, which constitutes 2% of NHLs with all cases having the translocation, the majority of NHLs with c-myc translocations are not BL. Some reported cases of ABL also lacked the c-myc translocation.[47,48]
Both (8;14)(q24;q32) and (14;18)(q32;q21) translocations can occur in the same malignant cells. Tumors harboring this combination have more aggressive disease, present in more advanced stages, and have a worse prognosis.[49,50] The t(14;18) causes overexpression of Bcl-2, which promote cell survival through apoptosis inhibition.[51,52] Dual translocation has been reported in BL cases, especially ABL,[49,53,54] but also DLBCL.[50] More complex cases of BL with triple translocations have been reported, and this has also been linked to poor prognosis.[54-56] The management approach in such complex translocation cases is not well defined. Whether “double-hit” DLBCL responds better to intensive therapy or CHOP-R (cyclophosphamide, doxorubicin HCl, vincristine [Oncovin], prednisone, rituximab [Rituxan]) is a question that has not yet been answered.
The pathogenesis of BL is not exclusively explained by c-myc dysregulation; other genetic aberrancies have been found to occur frequently. A p53 mutation was observed in 30% to 40% of BL cases in two reports,[57,58] and the 6q deletion was detected in 30% of cases in another study.[59]
Gene Profile Studies
Differentiating BL from DLBCL can be extremely difficult in some cases, but the distinction has important therapeutic implications. Gene-expression profiling was introduced in the past few years to compare gene expression based on the differential expression of thousands of genes measured simultaneously. Recent studies conducted by Dave et al[47] and Hummel et al[48] reported a more reliable approach in subclassification of mature aggressive B-cell lymphoma by gene-expression profiling using DNA microarrays.
These studies found that 17%[47] and 34%[48] of cases identified as having the Burkitt gene-expression profile had previously been classified as DLBCL or unclassifiable high-grade lymphoma by expert hematolpathologists, based on WHO criteria. On the contrary, 0.5% to 4% of the cases that had been called BL or ABL lacked the genetic signature of BL, which implies that these cases could represent DLBCL or a high-grade lymphoma rather than BL. Both studies revealed an occasional absence of the c-myc rearrangement in some cases. These studies proved the superiority of using gene signature in BL diagnosis over WHO criteria, although this is not easily applied in the clinic because of the complexities of the test. The results from the study by Dave et al showed a poor outcome for patients with the molecular signature of BL treated with CHOP-like regimens,[47] emphasizing the importance of this distinction.
Treatment
The initial trials of BL treatment by using standard protocols failed to obtain acceptable outcomes. When BL was treated with conventional NHL or ALL regimens, the complete response rate ranged from 30% to 70%, with cure rates between 0% and 30%.[1-4] This might be attributed to the ability of viable tumor cells to recover and reenter the cell cycle with a rapid growth rate between chemotherapy cycles. The introduction of intensive chemotherapy given over a relatively short interval has provided a strategy to address this problem. However, the enhancement of response came at the cost of increased treatment toxicity, which was an acceptable compromise in pediatric patients but problematic in adults, especially the elderly.
Most of the adult BL regimens have been adopted from pediatric protocols.[60-62] Although intensive chemotherapy improved outcomes markedly in pediatric BL, increased age was independently associated with inferior outcomes in most trials. Several pediatric regimens, such as the French LMB and German Multicenter Study Group for Adult ALL (GMALL) protocols, were attempted in adults early in the disease history. Both regimens achieved acceptable outcomes.
B-NHL 83 and B-NHL 86
The GMALL developed two protocols for adult BL derived from the pediatric Berlin-Frankfurt-Mnster (BFM) regimen. These protocols are B-NHL 83 (consisting of cyclophosphamide, prednisone, methotrexate, teniposide [Vumon], cytarabine, doxorubicin, and leucovorin, in six 5-day cycles) and B-NHL 86 (a similar regimen but substituting ifosfamide for cyclophosphamide in one phase, and dexamethasone for prednisone).[62] Trials investigating these protocols in adults reported 4- to 8-year overall survival rates of around 50%, which was a significant improvement compared to earlier outcomes. Nevertheless, toxicities were high with these regimens, including hematologic and neurologic toxicities, and 40% of patients could not complete the entire assigned regimen.
LMB Protocol
After the success that the LMB protocol had achieved in pediatrics, a retrospective review of 65 HIV-negative patients was conducted to evaluate its efficacy in the adult population. The LMB protocol includes a cytoreductive phase with the COP regimen (low dose cyclophosphamide, vincristine, and prednisone), followed by two induction cycles with COPADM (high dose of methotrexate, cyclophosphamide, vincristine, doxorubicin [Adriamycin], and prednisone), and then one to two consolidation cycles that contain cytarabine, and one to four maintenance cycles. The study involved previously untreated patients with small non–cleaved cell lymphoma or ALL L3. The majority of patients had advanced disease. Age ranged from 17 to 65. Approximately 89% of patients had a complete response, with a 3-year overall survival rate of 74%. The 3-year overall survival was 100% among patients with stage I/II disease, compared to 57% among patients with stage IV or ALL L3.[63] Subsequently, a prospective trial of LMB in adults was conducted on 51 patients (median age, 33). A complete response was achieved in 83% of patients, while 2-year event-free survival and overall survival were 61% and 66%, respectively. Multivariate analysis revealed an adverse outcome on survival rate associated with cytoreductive therapy failure and the presence of extranodal involvement.[64] A more recent phase II prospective trial of LMB in adults was published in 2005. The trial enrolled 72 adult patients with BL. A complete response rate of 72% was reported, with 2-year event-free and overall survival rates of 65% and 70%, respectively. Worse outcomes were noted in patients with increased lactate dehydrogenase level and older age. A 2-year overall survival of 84% was seen in patients aged < 33 years compared to 60% in patients > 33 years.[65] The most commonly reported adverse effect of the LMB protocol was myelosuppression.[63-65] Although employing the LMB protocol in adult BL has enhanced response and survival in this age group, the survival rate seen in pediatric patients was not achieved.
CODOX-M/IVAC
The CODOX-M/IVAC protocol, developed by Magrath, consists of alternating therapy between cyclophosphamide, vincristine (Oncovin), doxorubicin, high-dose methotrexate, plus intrathecal therapy, and ifosfamide, etoposide (VP-16), high-dose cytarabine (Ara-C), plus intrathecal therapy. Magrath and colleagues conducted a trial of four cycles of CODOX-M/IVAC on 41 patients, including children and adults, with small non–cleaved cell lymphoma. The study demonstrated a 2-year event-free survival rate of 92% in children as well as in adults. The investigators concluded that a similar prognosis can be obtained in adults and children when treated with CODOX-M/IVAC. However, the adults enrolled in this study were young (median age, 24). The reported major toxicities were mucositis and neurotoxicity.[61]
Two years later, Adde reported updated results obtained with four cycles of the alternating CODOX-M/IVAC protocol in advanced B-cell lymphomas. The regimen was given to 66 high-risk patients, including children and young adults; 55 patients had BL/BLL and 11 patients had DLBCL. The reported event-free survival was 85% at 1 year and beyond. Young adults were also included in this study (median age, 25).[66]
To establish the value of CODOX-M/IVAC protocol in adults, the UK Lymphoma Group conducted an international, prospective phase II study with some modification in the original protocol of CODOX-M/IVAC. The study enrolled 52 HIV-negative patients, with ages ranging from 16 to 60 years (median age, 35). Approximately 80% of these cases were considered high risk by International Prognostic Index–based criteria. Low-risk patients received three cycles of modified CODOX-M, whereas patients with high-risk parameters were given four cycles of alternating modified CODOX-M and IVAC. The 2-year overall and event-free survival rates for all patients were 73% and 65 %, respectively. In the low-risk group, 2-year overall and event-free survival rates were 82% and 83%, respectively, vs 70% and 60% in high-risk patients. The study revealed a trend of worsening event-free survival in older patients and those with advanced disease. Reported side effects were myelosuppression and mucositis.[67]
A similar outcome was obtained in a small prospective study using a modified Magrath regimen, performed by Dana-Farber Cancer Institute. The 2-year event-free survival was 64% in the entire study, with 100% in low-risk patients vs 60% in high-risk patients. However, less toxicity was seen with this modified regimen compared to Magrath’s original protocol.[68]
HyperCVAD With or Without Rituximab
Investigators at The University of Texas M.D. Anderson Cancer Center developed the HyperCVAD protocol, which includes hyperfractionated cyclophosphamide, vincristine, doxorubicin (Adriamycin), dexamethasone, and CNS prophylaxis. A trial was conducted in 26 adult BL patients with a median age of 58. The complete response rate was 81%, and the 3-year overall survival was 49%. The study was performed in relatively elderly patients compared to earlier discussed studies, with 46% of patients older than 60 years.[69]
Since Burkitt cells strongly express CD20 on the surface, and as rituximab is a monoclonal antibody against CD20, the introduction of rituximab to the preexisting regimens was investigated to improve survival in adult BL patients without worsening toxicity. A trial performed by Thomas et al revealed very promising results in elderly patients with BL. The study investigated the benefit of combining rituximab and HyperCVAD in 31 patients with BL. The median age was 46; 29% of patients were older than 60 years. The complete response rate was 86%, and the 3-year overall, event-free, and disease-free survival rates were 89%, 80%, and 88%, respectively. In patients older than 60 years, 3-year overall survival was 89%. In comparison to the HyperCVAD regimen in adults, HyperCVAD plus rituximab yielded a similar complete response rate but superior outcomes with regard to 3-year overall, event-free, and disease-free survival. The presence of CNS disease was not a prognostic factor in this study.[70]
NCCN Guidelines
The most recent National Comprehensive Cancer Network (NCCN) practice guidelines for NHL state that CHOP is an inadequate regimen for BL. Instead, the NCCN panel recommends using CODOX-M or HyperCVAD for the low-risk patient with BL, and CODOX-M/IVAC with or without rituximab, or HyperCVAD alternating with methotrexate plus cytarabine, with or without rituximab for the high-risk patient. The guidelines also note that stem cell transplantation should be considered for patients with relapsed disease.[71]
Bone Marrow Transplant
The role of autologous or allogeneic bone marrow transplant in adult BL is still controversial. Although some studies have reported that intensive therapy followed by stem cell transplant may improve survival,[72,73] it has not been accepted as a standard consolidation therapy. In a study investigating the efficacy of autologous transplants in adults with BL/ABL, a superior overall survival was obtained in treating relapsed chemosensitive disease. Compared to conventional regimens, the 3-year overall survival was 72% after the first complete remission, 37% in relapsed chemosensitive disease, and 7% in relapsed chemoresistant BL.[74]
In a more recent study of chemoradiotherapy and transplant as primary therapy in sporadic BL, the 3-year event-free survival rate was 50%.[73] The potential benefit of graft-vs-lymphoma by using allogeneic transplant affected neither the relapse rate nor overall survival based on current data.[75-77] The place of autologous and allogeneic transplant in BL remains unclear.
Other Treatment Considerations
Recently, selective serotonin-reuptake inhibitors (SSRIs) were reported to have an apoptotic effect on B-cell derived tumors, including BL.[78,79] More recent experiments failed to find a specific effect on either malignant cells or any particular subtype of cells. Therefore, SSRIs are unlikely to represent a potential modality in BL therapy.[80]
Central nervous system (CNS) prophylaxis is one of the main keys in BL treatment, since the CNS is a common site of relapse in the absence of such treatment. Prior to the introduction of intensive chemotherapy with CNS prophylaxis, CNS relapse occurred in 30% to 50% of patients with BL. High-dose systemic methotrexate or intrathecal methotrexate with or without cytarabine and hydrocortisone have been popular approaches to CNS prophylaxis.
Because of the rapid cell turnover in BL, physicians should be aware of the significant risk of tumor lysis syndrome, particularly in patients with extensive disease. The syndrome can be lethal by creating metabolic derangement (eg, hyperkalemia) and renal failure. It should be prevented by identifying patients at risk, administration of prophylactic allopurinol, correction of preexisting electrolyte disturbances, and maintenance of aggressive fluid hydration to keep a high urine output. Electrolytes should be closely monitored throughout the initial course of treatment.
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Allopurinol
Cyclophosphamide
Cytarabine
Dexamethasone
Doxorubicin
Hydrocortisone
Ifosfamide
Leucovorin
Methotrexate
Prednisone
Rituximab (Rituxan)
Teniposide (Vumon)
Vincristine
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Treatment of BL in HIV-positive patients is beyond the scope of this review. However, there is a worse prognosis for BL in HIV-positive patients compared to HIV-negative patients. The immunocompromised state of the HIV-positive patients has precluded the use of intensive chemotherapy in the past. However, the introduction of HAART in HIV management allowed the application of more intense therapy. Recent successful clinical trials using intensive regimens have been reported.[65,81-83] In contrast to the encouraging results that rituximab has produced in HIV-negative adults with BL, the role of rituximab in HIV-positive BL remains controversial.[84,85]
Conclusion
Dramatic advances continue to be made in the diagnosis and treatment of Burkitt lymphoma. Rapidly identifying the correct diagnosis is necessary for the provision of optimal therapy. Therefore, clear criteria for diagnosis and the use of new modalities such as gene-expression profiles may need to be involved in uncertain cases. Treatment of adult BL has improved remarkably in the past few years with the introduction of very intensive “pediatric” or “acute leukemia” protocols. Delay in therapy can have tragic results, but with rapid diagnosis and the prompt initiation of an appropriate treatment regimen the majority of adults will be cured.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Magrath IT, Janus C, Edwards BK, et al: An effective therapy for both undifferentiated (including Burkitt’s) lymphomas and lymphoblastic lymphomas in children and young adults. Blood 63:1102-1111, 1984.
2. Kantarjian HM, Walters RS, Keating MJ, et al: Results of the vincristine, doxorubicin, and dexamethasone regimen in adults with standard- and high-risk acute lymphocytic leukemia. J Clin Oncol 8:994-1004, 1990.
3. Bernasconi C, Brusamolino E, Pagnucco G, et al: Burkitt’s lymphoma/leukemia: A clinicopathologic study on 24 adult patients. Leukemia 5:90-94, 1991.
4. Ostronoff M, Soussain C, Zambon E, et al: Burkitt’s lymphoma in adults: A retrospective study of 46 cases. Nouv Rev Fr Hematol 34:389-397, 1992.
5. Burkitt DP: Sarcoma involving jaws in African children. Br J Surg 46:218-223, 1958.
6. Burkitt D, O’Conor GT: Malignant lymphoma in African children: I. Clinical syndrome. Cancer 14:258-269, 1961.
7. Murphy SB, Fairclough DL, Hutchison RE, et al: Non-Hodgkin’s lymphomas of childhood: An analysis of the histology, staging, and response to treatment of 338 cases at a single institution. J ClinOncol 7:186-193, 1989.
8. Diebold J, Jaffe E, Raphael M, et al: Burkitt lymphoma, in Jaffe E, Harris N, Stein H, et al (eds): Pathology and Genetics of Tumors of Hematopoietic and Lymphoid Tissues, 181-184. Lyon, France; IARC Press; 2001.
9. Cardy A, Sharp L, Little J: Burkitt’s lymphoma: A review of the epidemiology. Kuwait Med J 33:293-306, 2001.
10. Makata AM, Toriyama K, Kamidigo NO, et al: The pattern of pediatric solid malignant tumors in Western Kenya, East Africa, 1979-1994: An analysis based onhistopathologic study. Am J Trop Med Hyg 54:343-347, 1996.
11. Cairo MS, Sposto R, Perkins SL, et al: Burkitt’s and Burkitt-like lymphoma in children and adolescents: A review of the Children’s Cancer Group experience. Br J Haematol 120:660-670, 2003.
12. Agrath I: The pathogenesis of Burkitt’s lymphoma. Adv Cancer Res 55:133-270, 1990.
13. Blum KA, Lozanski G, Byrd JC: Adult Burkitt leukemia and lymphoma. Blood 104:3009-3020, 2004.
14. Boerma EG, van Imhoff GW, Appel IM, et al: Gender and age-related differences in Burkitt lymphoma-epidemiological and clinical data from The Netherlands. Eur J Cancer 40:2781-2787, 2004.
15. Sariban E, Donahue A, Magrath IT: Jaw involvement in American Burkitt’s lymphoma. Cancer 53:141-146, 1984.
16. Magrath IT, Sariban E: Clinical features of Burkitt’s lymphoma in the USA. IARC Sci Puble 60:119-127, 1985..
17. Hugh JC, Jackson FI, Hanson J, et al: Primary breast lymphoma. An immunohistologic study of 20 new cases. Cancer 66:2602-2611, 1990.
18. Burmeister T, Schwartz S, Horst HA, et al: Molecular heterogeneity of sporadic adult Burkitt-type leukemia/lymphoma as revealed by PCR and cytogenetics: Correlation with morphology, immunology and clinical features. Leukemia 19:1391-1398, 2005.
19. Gong JZ, Stenzel TT, Bennett ER, et al: Burkitt lymphoma arising in organ transplant recipients: A clinicopathologic study of five cases. Am J Surg Pathol 27:818-827, 2003.
20. Xicoy B, Ribera JM, Esteve J, et al: Post-transplant Burkitt’s leukemia or lymphoma. Study of five cases treated with specific intensive therapy (PETHEMA ALL-3/97 trial). Leuk Lymphoma 44:1541-1543, 2003.
21. Ferry JA: Burkitt’s lymphoma: Clinicopathologic features and differential diagnosis. Oncologist 11:375-383, 2006.
22. Knowles DM: Etiology and pathogenesis of AIDS-related non-Hodgkin’s lymphoma. Hematol Oncol Clin North Am 17:785-820, 2003.
23. Gabarre J, Raphael M, Lepage E, et al: Human immunodeficiency virus-related lymphoma: Relation between clinical features and histoloical subtypes. Am J Med 11:704-711, 2001.
24. Lim ST, Karim R, Nathwani BN, et al: AIDS-Related Burkitt’s lymphoma versus diffuse large-cell lymphoma in the pre-highly active antiretroviral therapy (HAART) and HAART eras: Significant differences in survival with standard chemotherapy. J Clin Oncol 23:4430-4438, 2005.
25. Sandler AS, Kaplan LD: Diagnosis and management of systemic non-Hodgkin’s lymphoma in HIV. Hematol Oncol Clin North Am 10:1111-1124, 1996.
26. Ostronoff M, Soussain C, Zambon E, et al: Burkitt’s lymphoma in adults: A retrospective studay of 46 cases. Nouv Rev Fr Hematol 34:389-397, 1992.
27. Harris N, Jaffe E, Diebold J, et al: World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee Meeting-Airlie House, Virginia, November 1997. J Clin Oncol 17:3835-3849, 1999.
28. Harris NL, Jaffe ES, Stein H, et al: A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 84:1361-1392, 1994.
29. Braziel RM, Arber DA, Slovak ML, et al: The Burkitt-like lymphomas: A Southwest Oncology Group study delineating phenotypic, genotypic, and clinical features. Blood 97:3713-3720, 2001.
30. Küppers R, Klein U, Hansmann M, et al: Cellular origin of human B-cell lymphomas. N Engl J Med 341:1520-1529, 1999.31. Jain R, Roncella S, Hashimoto S, et al: A potential role for antigen selection in the clonal evolution of Burkitt’s lymphoma. J Immunol 153:45-52, 1994.
32. Chapman CJ, Zhou JX, Gregory C, et al: VH and VL gene analysis in sporadic Burkitt’s lymphoma shows somatic hypermutation, intraclonal heterogeneity, and a role for antigen selection. Blood 88:3562-3568, 1996.
33. Chapman CJ, Wright D, Stevenson FK: Insight into Burkitt’s lymphoma from immunoglobulin variable region gene analysis. Leuk Lymphoma 30:257-267, 1998.
34. McClure RF, Remstein ED, Macon WR, et al: Adult B-cell lymphomas with Burkitt-like morphology are phenotypically and genotypically heterogeneous with aggressive clinical behavior. Am J Surg Pathol 29:1652-1660, 2005.
35. Dogan A, Bagdi E, Munson P, et al: CD10 and BCL-6 expression in paraffin sections of normal lymphoid tissue and B-cell lymphomas. Am J Surg Pathol 24:846-852, 2000.
36. Van der Burg M, Barendregt BH, van Wering ER, et al: The presence of somatic mutations in immunoglobulin genes of B cell acute lymphoblastic leukemia (ALL L3) supports assignment as Burkitt‘s leukemia-lymphoma rather than B-lineage ALL. Leukemia 15:1141-1143, 2001.
37. Macpherson N, Lesack D, Klasa R, et al: Small noncleaved, non-Burkitt’s (Burkitt-like) lymphoma: Cytogenetics predict outcome and reflect clinical presentation. J Clin Oncol 17:1558-1567, 1999.
38. Nakamura N, Nakamine H, Tamaru J, et al: The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod Pathol 15:771-776, 2002.
39. Haralambieva E, Boerma EJ, van Imhoff GW, et al: Clinical, immunophenotypic, and genetic analysis of adult lymphomas with morphologic features of Burkitt lymphoma. Am J Surg Pathol 29:1086-1094, 2005.
40. Magrath IT, Freeman CB, Bizzo P, et al: Characterization of lymphoma-derived cell lines: Comparison of cell lines positive and negative for Epstein-Barr virus nuclear antigen. II. Surface markers. J Natl Cancer Inst 64:64:477-483, 1980.
41. Hecht J, Aster J: Molecular biology of Burkitt’s lymphoma. J Clin Oncol 18:3703-3721, 2000.
42. Pelicci PG, Knowles DMd, Magrath I, et al: Chromosomal breakpoints and structural alterations of the c-myc locus differ in endemic and sporadic forms of Burkitt lymphoma. Proc Natl Acad Sci U SA 83:2984-2988, 1986.
43. Shiramizu B, Barriga F, Neequaye J, et al: Patterns of chromosomal breakpoint locations in Burkitt’s lymphoma: Relevance to geography and Epstein-Barr virus association. Blood 77:1516-1526, 1991.
44. Bhatia K, Spangler G, Gaidano G, et al: Mutations in the coding region of c-myc occur frequently in acquired immunodeficiency syndrome-associated lymphomas. Blood 84:883-888, 1994.
45. Joos S, Falk MH, Lichter P, et al: Variable breakpoints in Burkitt lymphoma cells with chromosomal t(8;14) translocation separate c-myc and the IgH locus up to several hundred kb. Hum Mol Genet 1:625-632, 1992.
46. Frost M, Newell J, Lones MA, et al: Comparative immunohistochemical analysis of pediatric Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Clin Pathol 121:384-392, 2004.
47. Dave SS, Fu K, Wright GW, et al: Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med 354:2431-2442, 2006.
48. Hummel M, Bentink S, Berger H, et al: A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med 354:2419-2430, 2006.
49. Thangavelu M, Olopade O, Beckman E, et al: Clinical, morphologic, cytogenetic characteristics of patients with lymphoid malignancies characterized by both t(14;18)(q32;q21) and t(8;14) (q24;q32) or t(8;22) (q24;q11). Genes Chromosomes Cancer 2:147-158, 1990.
50. Le Gouill S, Talmant P, Touzeau C, et al: The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica 92:1335-1342, 2007.
51. Nunez G, London L, Hockenberry D, et al: Deregulated bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol 144:3602-3610, 1990.
52. Hockenbery D, Nunez G, Milliman C, et al: Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348:334-336, 1990.
53. Karsan A, Gascoyne RD, Coupland RW, et al: Combination of t(14;18) and Burkitt’s type translocation in B-cell malignancies. Leuk Lymphoma 10:433-444, 1991.
54. Liu D, Shimonov J, Primanneni S, et al: t(8;14;18): A 3-way chromosome translocation in two patients with Burkitt’s lymphoma/leukemia. Mol Cancer 6:35, 2007.
55. Van Ooteghem RB, Smit EM, Beishuizen A, et al: A new B-cell line showing a complex translocation (8;14;18) and BCL2 rearrangement. Cancer Genet Cytogenet 74:87-94, 1994.
56. Zimonjic DB, Keck-Waggoner C, Popescu NC: Novel genomic imbalances and chromosome translocations involving c-myc gene in Burkitt’s lymphoma. Leukemia 15:1582-1588, 2001.
57. Preudhomme C, Dervite I, Wattel E: Clinical significance of p53 mutation in newly diagnosed Burkitt’s lymphoma and acute lymphoblastic leukemia: A report of 48 cases. J Clin Oncol 13:812-820, 1995.
58. Gaidano, G, Ballerini, P, Gong, JZ, et al: p53 mutations in human lymphoid malignancies: Association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 88:5413-5417, 1991.
59. Gaidano G, Hauptschein RS, Parsa NZ, et al: Deletions involving two distinct regions of 6q in B-cell non-Hodgkin lymphoma. Blood 80:1781-1787, 1992.
60. Bernstein JI, Coleman CN, Strickler JG, et al: Combined modality therapy for adults with small noncleaved cell lymphoma (Burkitt’s and non-Burkitt’s types). J Clin Oncol 4:847-858, 1986.
61. Magrath I, Adde M, Shad A, et al: Adults and children with small non-cleaved cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol 14:925-934, 1996.
62. Hoelzer D, Ludwig WD, Thiel E, et al: Improved outcome in adult B-cell acute lymphoblastic leukemia. Blood 87:495-508, 1996.
63. Soussain C, Patte C, Ostronoff M, et al: Small noncleaved cell lymphoma and leukemia in adults. A retrospective study of 65 adults treated with the LMB pediatric protocols. Blood 85:664-674, 1995.
64. Divine M, Cassasus P, Koscielny S, et al: Adult Burkitt lymphoma. A prospective multicenter trial with the LMB protocol (abstract 80). Proc Am Soc Clin Oncol 19, 2000.
65. Divine M, Casassus P, Koscielny S, et al: Burkitt lymphoma in adults: A prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol 16:1928-1935, 2005.
66. Adde M, Shad A, Venzon D, et al: Additional chemotherapy agents improve treatment outcome for children and adults with advanced B-cell lymphomas. Semin Oncol 25:33-39, 1998.
67. Mead GM, Sydes MR, Walewski J, et al: An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: Results of United Kingdom Lymphoma Group LY06 study. Ann Oncol 13:1264-1274, 2002.
68. LaCasce A, Howard O, Li S, et al: Modified Magrath regimens for adults with Burkitt and Burkitt-like lymphomas: Preserved efficacy with decreased toxicity. Leuk Lymphoma 45:761-767, 2004.
69. Thomas D, Cortes J, O’Brien S, et al: Hyper-CVAD program in Burkitt’s type adult acute lymphoblastic leukemia. J Clin Oncol 17:2461-2470, 1999.
70. Thomas DA, Faderl S,O’Brien S, et al: Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer 106:1569-1580, 2006.
71. NCCN clinical practice guidelines in oncology: Non-Hodgkin’s lymphoma, V.3.2008. Available at www.nccn.org. Accessed October 29, 2008.
72. Nademanee A, Molina A, O’Donnell MR, et al: Results of high-dose therapy and autologous bone marrow/stem cell transplantation during remission in poor-risk intermediate-and high-grade lymphoma: International index high and high-intermediate risk group. Blood 90:3844-3852, 1997.
73. Song, KW, Barnett, MJ, Gascoyne, RD, et al: Haematopoietic stem cell transplantation as primary therapy of sporadic adult Burkitt lymphoma. Br J Haematol 133:634-637, 2006.
74. Sweetenham J, Pearce R, Taghipour G, and et al: Adult Burkitt’s and Burkitt-like non-Hodgkin’s lymphoma-outcome for patients treated with high-dose therapy and autologous stem-cell transplantation in the first remission or at relapse: Results from the European Group for Blood and Marrow Transplantation. J Clin Oncol 14:2465-2472, 1996.
75. Grigg AP, Seymour JF: Graft versus Burkitt’s lymphoma effect after allogeneic marrow transplantation. Leuk Lymphoma 43:889-892, 2002.
76. Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al: An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: Allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant 31:667-678, 2003.
77. Ungkanont A, Mongkonsritrakoon W, Jootar1 S, et al: Allogeneic stem cell transplantation in a patient with refractory Burkitt’s lymphoma using non-myeloablative conditioning regimen. Bone Marrow Transplant 26:1351-1354, 2000.
78. Serafeim A, Holder MJ, Grafton G, et al: Selective serotonin reuptake inhibitors directly signal for apoptosis in biopsy-like Burkitt lymphoma cells. Blood 101:3212-3219, 2003.
79. Meredith EJ, Holder MJ, Chamba A, et al: The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: Probing a potential anti-tumor target for psychotropics. FASEB J 19:1187-1189, 2005.
80. Schuster C, Fernbach N, Rix U, et al: Selective serotonin reuptake inhibitors-a new modality for the treatment of lymphoma/leukaemia? Biochem Pharmacol 74:1424-1435, 2007.
81. Cortes J, Thomas D, Rios A, et al: Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone and highly active antiretroviral therapy for patients with acquired immunodeficiency syndrome-related Burkitt lymphoma/leukemia. Cancer 94:1492-1499, 2002.
82. Galicier L, Fieschi C, Borie R, et al: Intensive chemotherapy regimen (LMB86) for St Jude stage IV AIDS-related Burkitt lymphoma/leukemia: A prospective study. 110:2846-2854, 2007.
83. Wang ES, Straus DJ, Teruya-Feldstein J, et al: Intensive chemotherapy with cyclophosphamide, doxorubicine, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine (CODOX-M/ IVAC) for human immunodeficiency virus-associated Burkitt lymphoma. Cancer 98:1196-1205, 2003.
84. Kaplan LD, Lee JY, Ambinder RF, et al: Rituximab dose not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood 106:1538-1543, 2005.
85. Boue F, Gabarre J, Gisselbrecht C, et al: Phase II trial of CHOP plus rituximab in patients with HIV-associated non-Hodgkin’s lymphoma. J Clin Oncol 24:4123-4128, 2006.
86. Lopez T, Hagemeister F, McLaughlin P, et al: Small noncleaved cell lymphoma in adults: Superior results for stages I-III disease. J Clin Oncol 8:615-622, 1990.
87. McMaster M, Greer J, Greco A, et al: Effective treatment of small-noncleaved- cell lymphoma with high-intensity, brief-duration chemotherapy. J Clin Oncol 9:941-946, 1991.
88. Di Nicola M, Carlo-Stella C, Mariotti J, et al: Adult Burkitt’s lymphoma: High response rate with an intensive, short-term chemotherapy program (abstract 4741). Blood 100:300b, 2002.
89. Kujawski L, Longo WL, Williams EC, et al: High-dose CHOP and midcycle methotrexate for adult Burkitt and Burkitt-like lymphomas (abstract 3083). Blood 100:779a, 2002.