Management of Myelodysplastic Syndromes: 2008 Update
Myelodysplastic syndromes (MDS) are a heterogeneous collection of hematopoietic disorders that are thought to be clonal and are characterized by low blood counts and a tendency to progress to acute myeloid leukemia (AML).
ABSTRACT: Myelodysplastic syndromes (MDS) are a heterogeneous collection of hematopoietic disorders characterized by low blood counts and a tendency to progress to acute myeloid leukemia. Treatment options have increased over the past 10 years, and many new agents are currently being investigated. In this article, we review the diagnosis of MDS, prognostic systems, and treatment options, including supportive care, erythropoiesis-stimulating agents, antithymocyte globulin, lenalidomide, azacitidine, decitabine, intensive chemotherapy, and hematopoietic cell transplantation.
Myelodysplastic syndromes (MDS) are a heterogeneous collection of hematopoietic disorders that are thought to be clonal and are characterized by low blood counts and a tendency to progress to acute myeloid leukemia (AML). The incidence rate of MDS for 2001 to 2003 was 3.3/100,000, and the overall 3-year survival for MDS was 45%.[1] The incidence increases with age, and the median age of diagnosis is 70 to 75 years. Patients with MDS may be asymptomatic or may present with complaints related to cytopenias. The majority of patients have macrocytic anemia at presentation.
Diagnosis and Prognosis
The differential diagnosis of MDS includes other causes of macrocytic anemia such as vitamin B12 and folate deficiencies, alcohol consumption, and thyroid disorders. Initial laboratory workup includes blood counts, serum ferritin levels, total iron-binding capacity, serum iron, reticulocyte counts, vitamin B12 levels, red blood cell (RBC) folate levels, and thyroid-stimulating hormone levels. Persistent unexplained cytopenias warrant additional investigation with bone marrow aspiration and biopsy including cytogenetic testing and iron staining.
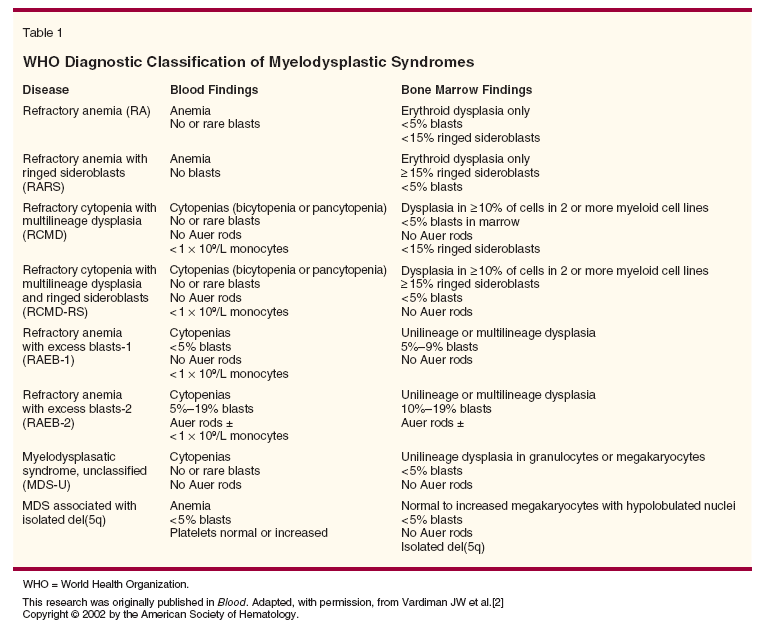
The diagnosis of MDS is based on the presence of dysplasia within single or multiple lineages (Table 1).[2] The World Health Organization (WHO) criteria distinguish between MDS and AML on the basis of a myeloblast percentage of ≥ 20%, thus eliminating the previous French-American-British (FAB)[3] diagnosis of refractory anemia with excess blasts in transformation (RAEB-T). Other important differences include the incorporation of multilineage dysplasia, which has been shown to impart an inferior prognosis, and the incorporation of isolated deletion of chromosome 5q, which is associated with improved survival. The WHO classification has proved helpful for prognosis[4] and in selection of therapy.[5] Despite advancements with the WHO classification, there is often limited concordance among pathologists in diagnosing lesser degrees of dysplasia, and the diagnosis may be delayed for months following initial presentation.
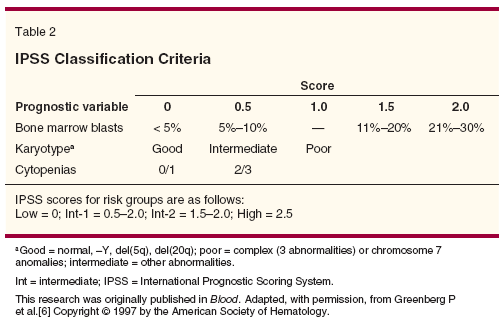
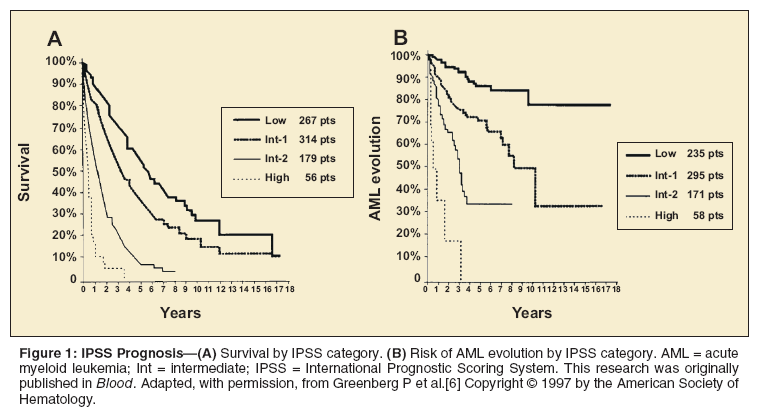
The International Prognostic Scoring System (IPSS) was developed on the basis of 816 patients with primary MDS.[6] Investigators found that myeloblast percentage, cytogenetic status, and number of low blood counts offer prognostic information (Table 2). The IPSS divided patients into four risk groups (low, intermediate [int]-1, int-2, and high) and reliably discriminated between these groups in survival and AML progression (Figure 1). The limitations of the IPSS are that it is only applicable using data from the time of diagnosis and that it is not applicable to patients with secondary MDS.
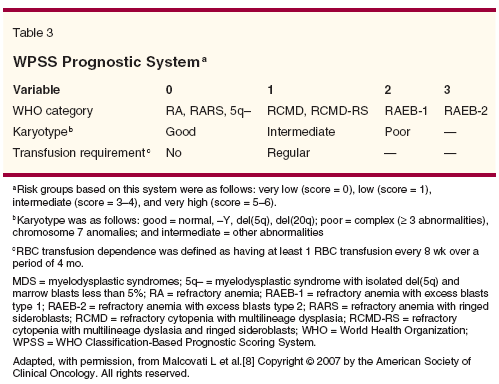
Recent data have shown that transfusion burden is associated with inferior survival in MDS
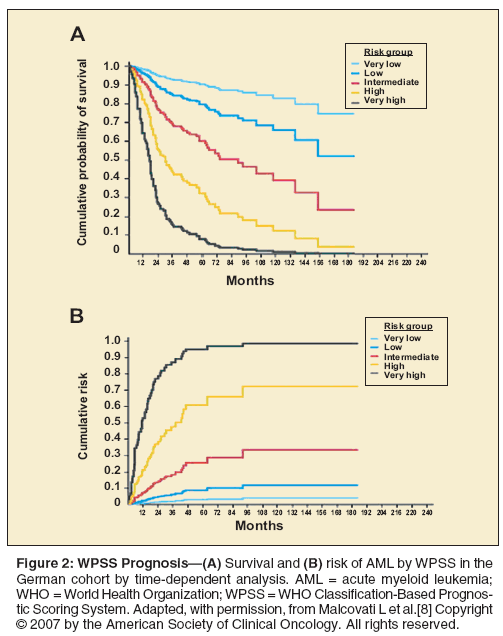
patients.[7] On the basis of this finding, a new prognostic system-the WHO Classification-Based Prognostic Scoring System (WPSS)-incorporated the WHO classification, transfusion dependence, and IPSS cytogenetic status (Table 3).[8] Patients with secondary MDS were also excluded from the WPSS. The advantage of the WPSS is that it provides a real-time assessment of prognosis (Figure 2). Whether the WPSS offers an advantage over IPSS classifications with regard to prognosis at time of diagnosis is not yet known.
A recently validated flow cytometric scoring system (FCSS) may add information regarding prognosis, particularly in patients who are otherwise thought to be at low risk.[9] Flow cytometry may be helpful with both diagnosis and prognosis.
Treatment of MDS
As described above, the term MDS incorporates a broad and heterogeneous group of disorders. Not surprisingly, it is difficult to recommend a routine method of determining the appropriate course of action in all patients. Therefore, appropriate therapeutic choices are influenced by factors such as patient preference, performance status, cost, cytogenetic status, tempo of blood count decline, and patient age.
Supportive Care
Patients who are asymptomatic with low-risk disease and stable blood counts may not require any treatment. The main goal in these patients includes close monitoring of blood counts and frequent marrow aspirations to follow the disease course. We recommend that asymptomatic patients with low-risk disease have yearly marrow evaluations and monthly blood counts.
• Transfusion and Hematopoiesis-Patients who have symptomatic cytopenias with coexisting medical illnesses that would prevent compliance with therapy may benefit from limited transfusion support and antibiotics as indicated. There is no single hemoglobin cutoff at which RBC transfusion support should be offered to patients. Rather, decisions regarding RBC transfusion support should be made on an individual basis. At our center, we routinely offer platelet transfusions when the platelet count is < 10,000/µL, but we do adjust this based on individual risk factors and bleeding tendencies.
MDS is a disease not only of decreased production but also of abnormal function. Therefore, patients may have symptoms that appear to be out of proportion to the level of cytopenia.[10,11] Granulocyte colony-stimulating factor (G-CSF, Neupogen) may be useful in neutropenic patients with recurrent or resistant infections but should not be used for routine infection prophylaxis.[12,13]
• Iron Chelation-Iron chelation remains controversial. While increased ferritin is associated with inferior survival,[7] it is not clear that reduction in ferritin leads to improved survival. Retrospective cohort studies demonstrated an improved survival in patients who received iron chelation.[14] However, a selection bias confounds these retrospective studies, given that many patients receive iron chelation because they are expected to have a longer survival.
The current National Comprehensive Cancer Network (NCCN) guidelines recommend considering iron chelation in patients who have received more than 20 to 30 units of RBC transfusions. Other considerations are the serum ferritin and the patient’s anticipated life expectancy. Since complications from iron overload can take years to develop, some experts argue that patients with a short life expectancy (< 1 year) should not receive iron chelation therapy.
Deferasirox (Exjade) is US Food and Drug Administration (FDA)-approved for chronic iron overload due to RBC transfusions in patients who are ≥ 2 years old. It is important to assess liver function monthly in patients who receive deferasirox, as hepatic dysfunction has been described in postmarketing surveillance reports. Auditory and ophthalmic testing is also recommended prior to and yearly thereafter in patients receiving deferasirox.
Erythropoiesis-Stimulating Agents
Although erythropoiesis-stimulating agents (ESA) are not FDA-approved for MDS, ironically they are the most commonly used agents in this setting. ESA have been associated with a shorter survival in patients with solid malignancies in a phase III randomized placebo-controlled study.[15] Given the emergence of these survival data, the Oncologic Drugs Advisory Committee (ODAC) met On March 13, 2008, to discuss the continued use of ESA in patients with malignancies. ODAC recommended continued marketing of ESA for chemotherapy-induced anemia, but it requested amendments to the label stating that ESA are not indicated for patients receiving potentially curative therapy nor for patients with metastatic breast or head/neck cancer.
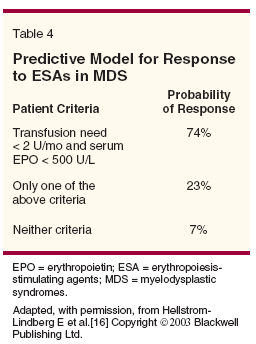
A validated decision model uses baseline erythropoietin level and previous RBC transfusion requirements to predict response to ESA (Table 4).[16] ESA are most effective for low- or int-1–risk MDS patients with < 5% myeloblasts.[17,18]
A phase III randomized, placebo-controlled study did not show a statically significant improvement in hemoglobin or reduction in RBC transfusion requirements with ESA plus granulocyte-macrophage colony-stimulating factor (GM-CSF, Neupogen).[13] However, there were only 37 patients with an erythropoietin level ≤ 500 mU/mL included in this trial. Therefore, the lack of a significant improvement with ESA plus GM-CSF is likely a reflection of the low power to detect a benefit rather than an absence of benefit.
Another phase III randomized study in 118 patients with MDS did show a significant improvement in hemoglobin and reduction in RBC transfusion requirements in patients treated with epoetin alfa (Epogen, Procrit) at 150 U/kg/d (35% vs 9%, P = .002).[19] Recently, two separate investigative groups performed retrospective analyses comparing MDS patients treated with ESA to MDS patients who received no treatment. These studies suggested a survival benefit in patients treated with ESA.[18,20]
The primary weaknesses of these analyses are the retrospective nature and the potential lack of comparability between the cohorts. This is highlighted by a previous study that found no survival benefit comparing ESA-treated patients to untreated patients. The untreated cohort was taken from the same database used in the French study.[17]
Despite these conflicting results, ESA improve symptoms of anemia, increase hemoglobin levels, and decrease transfusion requirements in patients with low-risk or int-1–risk MDS. The recommended dose of epoetin alfa is 40,000 to 60,000 units 1 to 3 times per week, and the recommended dose of darbepoetin alfa (Aranesp) is 150 to 300 µg/wk. Patients should receive ESA for at least 8 weeks before a decision is made regarding effectiveness. Studies demonstrate conflicting results regarding G-CSF and ESA synergistically stimulating erythropoiesis,[21,22] and we do not routinely combine G-CSF with ESA.
Antithymocyte Globulin
In a phase II study, 61 patients with RBC transfusion-dependent MDS received horse antithymocyte globulin (ATG, Atgam) at 40 mg/kg/d for 4 days.[23] A total of 21 patients (34%) became transfusion-independent with a median response time of 10 weeks. ATG responders had a longer survival and a lower risk of disease progression than nonresponders. Younger age, overrepresentation of HLA-DR 15, and a shorter duration of RBC transfusion dependence is associated with response to ATG.[24]
In a randomized phase II study, horse ATG at 15 mg/kg/d for 5 days was compared to rabbit ATG (Thymoglobulin) at 3.75 mg/kg/d for 5 days in MDS patients with clinically significant cytopenias.[25] The investigators observed no difference in response rates (42%). Recently, National Heart, Lung, and Blood Institute (NHLBI) researchers retrospectively compared MDS patients treated with ATG to patients who had received no therapy (International Myelodysplasia Risk Analysis Workshop [IMRAW] database). They demonstrated that patients who received ATG had a lower risk for death (hazard ratio [HR] = 0.5; P = .002) and lower risk for progression to AML (HR = 0.45; P = .092).[26] This analysis compared patients from different time periods and from different centers. The majority of ATG studies were performed at a single center, and repeating similar studies at other institutions has been complex.[27]
Lenalidomide
Lenalidomide (Revlimid) is FDA-approved for MDS patients who have low- or int-1–risk disease by the IPSS with associated deletion of 5q and who are transfusion-dependent. In a multicenter phase II study, MDS patients with del(5q) were treated with 10 mg/d of lenalidomide given orally.[28] Patients with an absolute neutrophil count < 500/µL and a platelet count < 50,000/µL were excluded. A total of 148 patients were treated for 24 weeks, at which point disease was assessed with bone marrow aspiration, biopsy, and cytogenetic evaluation. The primary endpoint of the study was transfusion independence.
The chief toxicity was neutropenia and thrombocytopenia; in fact, 79% of patients enrolled in this study required dose reductions secondary to hematopoietic toxicity. Thus, patients should have weekly blood counts for the first 8 weeks following initiation of lenalidomide. Patients should be treated for at least 12 weeks before making a decision regarding effectiveness. Among the 148 patients enrolled, 112 had reduced transfusion requirements and 99 (67%) became transfusion-independent with a median response time of 4.6 weeks. The median duration of transfusion independence was not reached at the time of publication.
In a simultaneous multicenter phase II study, 214 transfusion-dependent MDS patients with low- or int-1–risk MDS without del(5q) were treated with lenalidomide, 10 mg/ d.[29] The most commonly observed grade 3/4 adverse events were thrombocytopenia (25%) and neutropenia (30%). A total of 56 patients (26%) achieved transfusion independence after a median of 4.8 weeks of treatment, with a median duration of transfusion independence of 41 weeks. The use of lenalidomide in more advanced stages of MDS, such as patients with ≥ 10% myeloblasts, is an area of ongoing investigation. Whether lenalidomide affects survival or progression to AML is unknown.
Azacitidine

Azacitidine (Vidaza) is FDA-approved for MDS patients in all IPSS categories and all FAB subtypes. A phase III multicenter trial randomized 191 MDS patients to azacitidine (75 mg/m2/d × 7days every 28 days) or supportive care (transfusions and antibiotics).[30,31] The overall response rate was 60%, with 7% achieving a complete remission and 16% partial remission. The major limitation of the study is that crossover was allowed, and approximately half of the patients (n = 49) initially randomized to supportive care crossed over to azacitidine therapy prior to 6 months following enrollment. In essence, therefore, the study compared azacitidine upfront to azacitidine later.
Not surprisingly, the study was unable to show a difference in overall survival. However, a landmark analysis was performed that did show a survival benefit with azacitidine when patients who crossed over to azacitidine prior to 6 months after enrollment were excluded from the analysis (median survival = 11 vs 18 months; P = .03). Additionally, the investigators observed a significant delay in progression to leukemia or death in patients who received azacitidine (median = 12 vs 21 months; P = .007).
The major toxicity observed in this trial was myelosuppression, with grade 3 or 4 granulocytopenia in 43% and thrombocytopenia in 52%. The median time to response was 64 days, and the median duration of response was 15 months. Patients should receive at least six cycles of azacitidine prior to deciding effectiveness. Blood counts may initially decline with azacitdine, particularly during the first two cycles.
Azacitidine survival data in a multicenter phase III study were presented at the 2007 American Society of Hematology annual meeting. However, these findings have not yet been published in a peer-reviewed journal.
Decitabine
Decitabine (Dacogen) is FDA-approved for int-1–, int-2–, and high-risk MDS patients with all FAB subtypes. In a phase III multicenter trial, 170 patients with MDS were randomized to decitabine (15 mg/m2 every 8 hours for 3 days every 6 weeks) or supportive care (transfusions, antibiotics, and growth factors).[32] The overall response rate with decitabine was 17%, with 9% complete remissions and 13% hematologic improvements. Patients who received decitabine showed a trend toward delay in progression to AML or death (median = 12.1 vs 7.8 months; P = .16).This trend was significant in patients with int-2– or high-risk disease (median = 12.6 vs 9.4 months; P = .03). However, the investigators found no difference between decitabine therapy and supportive care in overall survival.
A subsequent study randomized 95 MDS patients with int-1–, int-2–, or high-risk disease to decitabine at 20 mg/m2/d IV for 5 days, decitabine at 20 mg/m2/d subcutaneously for 5 days, or decitabine at 10 mg/m2/d IV for 10 days.[33] Notably, the FDA-approved dose was not included in this analysis. A Bayesian design was used for this protocol, allowing for adaptive randomization with a prior pretest probability distributed equally among the three regimens. A total of 64 patients received 20 mg/m2/d IV for 5 day, 14 received 20 mg/m2/d subcutaneously for 5 days, and 17 received 10 mg/m2/d IV for 10 days. Decitabine at 20 mg/m2/d IV for 5 days was the optimal regimen, with a complete remission rate of 39% vs 21% vs 24% (P < .05), respectively.
A large study by the European Organisation for Research and Treatment of Cancer (EORTC), randomizing MDS patients to decitabine vs supportive care with a primary endpoint of survival, has completed accrual.
Intensive Chemotherapy
Very few published studies evaluating intensive chemotherapy are focused solely on MDS. Rather, most publications have evaluated AML and “high-risk” MDS together with variable definitions of “high-risk” MDS not based on the IPSS. Therefore, it is difficult to evaluate intensive chemotherapy in MDS based on published literature.
Investigators at M.D. Anderson Cancer Center have published data suggesting that topotecan (Hycamtin)-based chemotherapy may be preferable in patients with MDS.[34] In a retrospective analysis, 77 patients received topotecan/cytarabine, 270 received idarubicin/cytarabine, 67 received topotecan/cytarabine and cyclophosphamide, and 96 received fludarabine/cytarabine. Topotecan/cytarabine regimens were equivalent to idarubicin/cytarabine regimens with regard to complete response and survival rates but were associated with lower induction mortality rates.
A topical issue concerns how the introduction of hypomethylating agents affects the more conventional option of intensive chemotherapy. Proponents of hypomethylating agents argue that they have a lower toxicity profile, allowing patients to receive therapy on an outpatient basis, and that the lower complete response rates do not necessarily translate into lower survival rates. This may be particularly relevant for older MDS patients, who are at higher risk of morbidity from intensive chemotherapy.
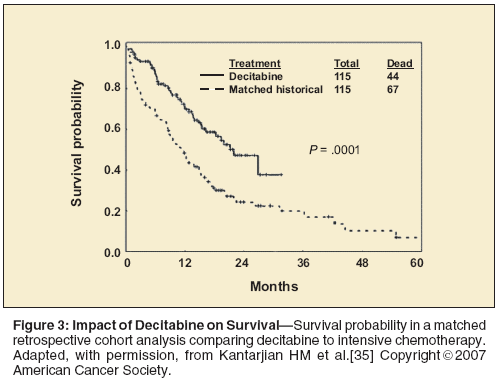
To date, no prospective randomized study has evaluated hypomethylating agents against intensive chemotherapy. However, a retrospective matched-cohort analysis indicated a survival advantage with decitabine (n = 115) in comparison to intensive chemotherapy (n = 115)-median survival = 22 vs 12 months; P < .001 (Figure 3).[35] Matching parameters included age, chromosomal abnormalities, and IPSS risk group. The major limitations of the analysis were its retrospective nature and the lack of comparable time periods of treatment (2001–2003 for the decitabine cohort but starting in 1995 for the intensive chemotherapy cohort). Another limitation of this analysis is that there were a variety of regimens included in the intensive chemotherapy cohort (topotecan, clofarabine [Clolar], fludarabine, anthracyclines, etc). Despite these limitations, the study was the first attempt to address this emerging issue in the treatment of MDS patients.
At our center, we tend to use intensive chemotherapy regimens in MDS patients with > 10% marrow myeloblasts who are candidates for stem-cell transplantation.
Hematopoietic Cell Transplantation
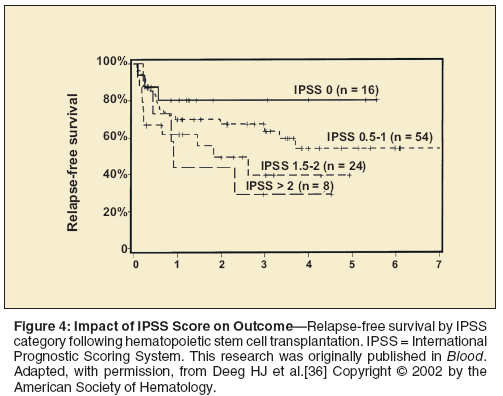
Hematopoietic cell transplantation (HCT) has curative potential for patients with MDS (Figure 4).[36] Unfortunately, most patients with MDS are not eligible for HCT because of a lack of donor availability or because of advanced comorbidity scores.[37] The major limitation of HCT is the upfront treatment-related mortality, which can vary from 20% to 30% depending on patient- and donor-specific factors. Since low- or int-1–risk MDS patients can live for many years without disease progression, some experts question the utility of HCT in MDS patients historically considered “good risk.”
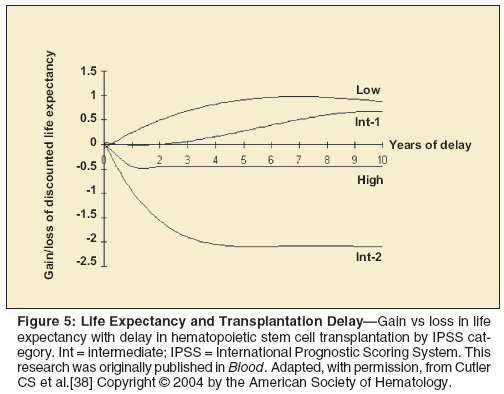
A multicenter retrospective analysis used a Markov decision model to evaluate the optimal timing of HCT in MDS.[38] Based on this analysis, low- or int-1–risk MDS patients benefit from a delay in HCT, whereas int-2– or high-risk MDS patients should be offered HCT at diagnosis (Figure 5). Reduced-intensity conditioning regimens are an emerging issue in the field of HCT. These regimens are less toxic but are also associated with a higher relapse risk.[39] Retrospective analysis comparing conventional regimens to reduced-intensity conditioning regimens show no difference in survival.[40] However, this has not been confirmed in a randomized study.
Summary
Ten years ago, there was little to offer MDS patients other than transfusion support, but since then, treatment options have increased. Furthermore, many new agents are being investigated. The holy grail of MDS treatment remains targeted therapy directed at specific pathogenic mechanisms. Since there is not one universal pathogenic mechanism that operates in all cases of MDS, it is unlikely that a single agent will be applicable to all patients. It will be important to refine MDS classification to reflect the pathogenic mechanisms operating, so that clinical trials will be directed to appropriate subtypes. It is only through enrolling patients into clinical trials that our understanding of MDS and the underlying pathogenic mechanisms will advance.
References:
References
1. Rollison DE, Howlader N, Smith MT, et al: Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood 112:45-52, 2008.
2. Vardiman JW, Harris NL, Brunning RD: The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100:2292-2302, 2002.
3. Bennett JM, Catovsky D, Daniel MT, et al: Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 51:189-199, 1982.
4. Malcovati L, Porta MG, Pascutto C, et al: Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: A basis for clinical decision making. J Clin Oncol 23:7594-7603, 2005.
5. Howe RB, Porwit-MacDonald A, Wanat R, et al: The WHO classification of MDS does make a difference. Blood 103:3265-3270, 2004.
6. Greenberg P, Cox C, LeBeau MM, et al: International scoring system for evaluating prognosis in myelodysplastic syndromes (erratum appears in Blood 91:1100, 1998). Blood 89:2079-2088, 1997.
7. Malcovati L, Della Porta MG, Cazzola M: Predicting survival and leukemia evolution in patients with myelodysplastic syndrome. Haematologica 91:1588-1590, 2006.
8. Malcovati L, Germing U, Kuendgen A, et al: Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 25:3503-3510, 2007.
9. Scott BL, Wells DA, Loken MR, et al: Validation of a flow cytometric scoring system as a prognostic indicator for post-transplant outcome in patients with MDS. Blood July 7 2008 (epub ahead of print).
10. Yamaguchi N, Ito Y, Ohyashiki K: Increased intracellular activity of matrix metalloproteinases in neutrophils may be associated with delayed healing of infection without neutropenia in myelodysplastic syndromes. Ann Hematol 84:383-388, 2005.
11. Zeidman A, Sokolover N, Fradin Z, et al: Platelet function and its clinical significance in the myelodysplastic syndromes. Hematol J 5:234-238, 2004.
12. Negrin RS, Haeuber DH, Nagler A, et al: Treatment of myelodysplastic syndromes with recombinant human granulocyte colony-stimulating factor. A phase I-II trial. Ann Intern Med 110:976-984, 1989.
13. Thompson JA, Gilliland DG, Prchal JT, et al: Effect of recombinant human erythropoietin combined with granulocyte/macrophage colony-stimulating factor in the treatment of patients with myelodysplastic syndrome. Blood 95:1175-1179, 2000.
14. Leitch HA: Improving clinical outcome in patients with myelodysplastic syndrome and iron overload using iron chelation therapy. Leuk Res 31(suppl 3):S7-S9, 2007.
15. Smith RE Jr, Aapro MS, Ludwig H, et al: Darbepoetin alfa for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: Results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol 26:1040-1050, 2008.
16. Hellstrom-Lindberg E, Gulbrandsen N, Lindberg G, et al: A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: Significant effects on quality of life. Br J Haematol 120:1037-1046, 2003.
17. Jädersten M, Montgomery SM, Dybedal I, et al: Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood 106:803-811, 2005.
18. Park S, Grabar S, Kelaidi C, et al: Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: The GFM experience. Blood 111:574-582, 2008.
19. Miller KB, Kim HT, Greenberg P, et al: Phase III prospective randomized trial of EPO with or without G-CSF versus supportive therapy alone in the treatment of myelodysplastic syndromes (MDS): Results of the ECOG-CLSG trial (E1996) (abstract 70). Blood 104:24a, 2004.
20. Jädersten M, Malcovati L, Dybedal I, et al: Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol 26:3607-3613, 2008
21. Park S, Grabar S, Kelaidi C, et al: Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: The GFM experience. Blood 111:574-582, 2008.
22. Hellstrom-Lindberg E, Ahlgren T, Beguin Y, et al: Treatment of anemia in myelodysplastic syndromes with granulocyte colony-stimulating factor plus erythropoietin: Results from a randomized phase II study and long-term follow-up of 71 patients. Blood 92:68-75, 1998.
23. Molldrem JJ, Leifer E, Bahceci E, et al: Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndrome [summary for patients in Ann Intern Med 137:I-27, 2002]. Ann Intern Med 137:156-163, 2002.
24. Saunthararajah Y, Nakamura R, Wesley R, et al: A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood 102:3025-3027, 2003.
25. Stadler M, Germing U, Kliche KO, et al: A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia 18:460-465, 2004.
26. Sloand EM, Wu CO, Greenberg P, et al: Factors affecting response and survival in patients with myelodysplasia treated with immunosupporessive therapy. J Clin Oncol 26:2505-2511, 2008.
27. Steensma DP, Dispenzieri A, Moore SB, et al: Antithymocyte globulin has limited efficacy and substantial toxicity in unselected anemic patients with myelodysplastic syndrome. Blood 101:2156-2158, 2003.
28. List A, Dewald G, Bennett J, et al: Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 355:1456-1465, 2006.
29. Raza A, Reeves JA, Feldman EJ, et al: Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1-risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood 111:86-93, 2008.
30. Silverman LR, Demakos EP, Peterson BL, et al: Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the Cancer and Leukemia Group B. J Clin Oncol 20:2429-2440, 2002.
31. Silverman LR, McKenzie DR, Peterson BL, et al: Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol 24:3895-3903, 2006.
32. Kantarjian H, Issa JP, Rosenfeld CS, et al: Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer 106:1794-1803, 2006.
33. Kantarjian H, Oki Y, Garcia-Manero G, et al: Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 109:52-57, 2007.
34. Kantarjian H, Beran M, Cortes J, et al: Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer 106:1099-1109, 2006.
35. Kantarjian HM, O’Brien S, Huang X, et al: Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: Comparison with historical experience. Cancer 109:1133-1137, 2007.
36. Deeg HJ, Storer B, Slattery JT, et al: Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood 100:1201-1207, 2002.
37. Sorror ML, Sandmaier BM, Storer BE, et al: Comorbidity and disease status-based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol 25:4246-4254, 2007.
38. Cutler CS, Lee SJ, Greenberg P, et al: A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: Delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 104:579-585, 2004.
39. Martino R, Iacobelli S, Brand R, et al: Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood 108:836-846, 2006.
40. Scott BL, Sandmaier BM, Storer B, et al: Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: A retrospective analysis. Leukemia 20:128-135, 2006.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.