Advanced Urothelial Cancer: Updates in Frontline Systemic Therapy
CancerNetwork® recap of an Around the Practice event with Neeraj Agarwal, MD, Petros Grivas, MD, PhD, Guru Sonpavde, MD, Thomas Powles, MBBS, MD, MRCP
Petros Grivas, MD, PhD, Fred Hutchinson Cancer Research Center, Seattle, WA

Guru P. Sonpavde, MD, Dana-Farber Cancer Institute, Boston, MA

Thomas Powles, MBBS, MD, Barts Cancer Centre, London, UK

Neeraj Agarwal, MD, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT

The treatment of urothelial cancer can present challenges given the tendency for patients with early-stage tumors to experience rapid progression to advanced disease following standard management. Additionally, recent data on the use of immunotherapy in the frontline setting have left many wondering where to place that category of agents in the treatment paradigm.
Experts discussed these topics in a virtual CancerNetwork® Around the Practice presentation, “Advanced Urothelial Cancer,” held on July 14, 2021, and moderated by Petros Grivas, MD, PhD. Members of the audience also participated in an interactive online platform by submitting responses to polling questions that were subsequently discussed by the panelists.
Typical Presentation of Urothelial Carcinoma
When polled about the patients treated in their clinics, most respondents indicated that between 30% and 40% of their practice consists of patients who have locally advanced or metastatic urothelial cancer. In the discussion following the poll, the panel discussed risk factors for the disease and typical patient presentation at the time of diagnosis.
Neeraj Agarwal, MD,noted common risk factors in the United States that he reviews with all his trainees, including White race, male sex, and having environmental and social risk factors such as exposure to hydrocarbons and a smoking history. Thomas Powles, MBBS, MD, pointed out that in his practice in the United Kingdom, metastatic disease is often seen in the clinic following progression from an earlier setting, also speaking to the long patient journey in this disease.
The discussion then shifted to disease prognosis in these patients, with physical functioning and assessment standards such as the Bajorin criteria, defined as performance status plus the presence of visceral metastases, which have come into play for most of the panel.1 Powles noted specifically that patients with node-only disease in the absence of visceral metastases do well irrespective of systemic therapy. Other factors influencing treatment success include renal function, cisplatin eligibility, and albumin levels.
Diagnostic and Treatment Challenges
As the panel moved forward in their discussion, they noted that diagnosis of both early and metastatic disease can sometimes be quite challenging, because early indicators of disease tend to be nonspecific to bladder cancer.
“Urinary tract infections [UTIs] are of course a much more common cause of dysuria. You can have kidney stones suspected, so it’s important [in] somebody who has long-standing symptoms [that] they keep coming back [for monitoring],” said Guru Sonpavde, MD. “Start suspecting bladder cancer when you see hematuria, even microscopic hematuria, because this cancer does colonize in the lining of the bladder and does tend to cause hematuria early on. That is something to be vigilant about; voiding symptoms are not always UTIs or infections, so always be mindful of that, especially in women. It tends to get misdiagnosed frequently as UTIs for a while.”
Considering systemic treatment, Agarwal discussed a study he coauthored. The results indicated that less than half of patients with metastatic disease treated in the frontline setting received this care and that about 15% to 20% received it as second-line therapy.2
“These data were based on the studies done until 2018, so we did not have enfortumab vedotin [Padcev] or sacituzumab govitecan [Trodelvy], which were just approved [by the FDA in 2019 and 2020, respectively]. But PD-1/PD-L1 inhibitors were already approved in the United States, so these data were alarming to me,” Agarwal said.
Guidelines Recommendations
Per professional guidelines such as those from the National Comprehensive Cancer Network (NCCN)3 and the European Society for Medical Oncology, treatment choice in the frontline setting typically hinges on whether the patient is fit for cisplatin-based chemotherapy, which has the highest level of evidence in this patient population. In patients who cannot receive the frontline standard of gemcitabine plus cisplatin, gemcitabine plus carboplatin is an acceptable treatment alternative.
“The reality is that both of these [doublets], in recent…randomized studies, [demonstrated similar] response rates: between 45% and 55% for gemcitabine plus cisplatin and between 45% and 50% for gemcitabine plus carboplatin,” Powles said. “Median progression-free survival for both regimens [is about] 6 to 8 months, and overall survival for both regimens is in the area of 12 to 14 months.”
Another patient subgroup that has emerged includes those who are not eligible for cisplatin and highly express PD-L1. For them, atezolizumab (Tecentriq) and pembrolizumab (Keytruda) are approved and supported by level 3b evidence per the NCCN.3-5
“[Approval was based] off single-arm trials at relatively modest numbers. The original trials had an all-comer population, but the labels were [later] restricted when the randomized trials were performed,” said Powles.6 Those randomized trials didn’t show or haven’t yet shown an advantage for immune checkpoint inhibitors [ICIs] over standard chemotherapy. What we’ve learned is that if you give up-front ICIs, the response rates [are] lower than [with] chemotherapy. Many patients progress, and then you don’t get the opportunity to sequence in other drugs.”
Continuing with the topic of placement of immunotherapy in the frontline setting, Powles pivoted the discussion to maintenance therapy with the PD-L1 antibody avelumab (Bavencio).
“The global community currently [considers] the standard of care [to be] gemcitabine [plus cisplatin or carboplatin], followed by maintenance avelumab in those patients who are not progressing,” he said.
Grivas then posed a question to the audience about their preferred management of patients who are not eligible for cisplatin in the frontline setting but have high PD-L1 expression. Between carboplatin plus gemcitabine or an ICI, about 75% of respondents said they would opt to stick with chemotherapy. The panel agreed that if a patient such as this presented to their practice, the preferred course of treatment would involve frontline chemotherapy with avelumab maintenance.
Maintenance Therapy
When polled about the role of frontline maintenance in advanced urothelial carcinoma, 63% of respondents said they offer it to all their patients and another 27% reported using it in certain patients (Figure).
FIGURE. AUDIENCE POLL: Do you think first-line maintenance therapy has a role in the management of advanced urothelial carcinoma?
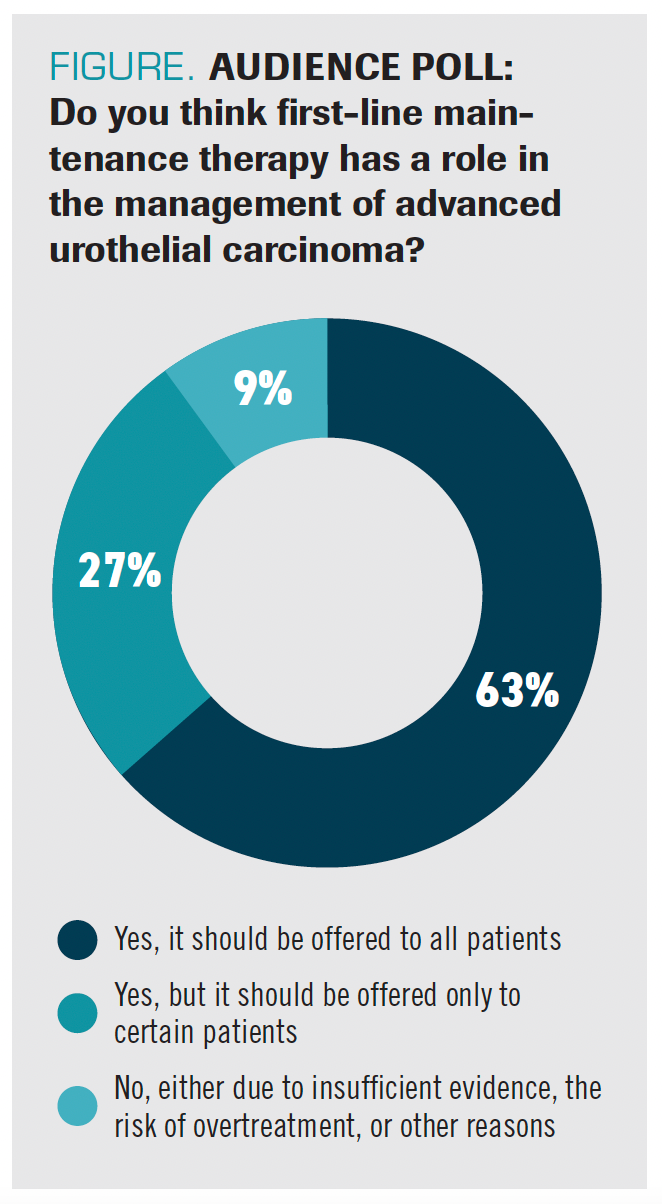
“Patients do well with chemotherapy, but resistance occurs very quickly and there’s a real frustration,” said Powles. “Second-line therapy is too late for most patients—even those patients who get second-line chemotherapy or second-line ICIs. When the cancer [is] growing fast, the principle is, can we just maintain that control?”
In discussing the evolution of maintenance therapy in this patient population, Powles then pointed to a study he coauthored on the use of lapatinib (Tukysa) maintenance in those with HER1/HER2 overexpression.7 That was among the first trials to examine this principle, although the end points remained unreached. However, these results did not shut the door on the principle of maintenance therapy altogether.
“When the ICIs came out, it became apparent that second-line immune therapy with pembrolizumab was too late for most…and first-line ICIs didn’t get it under control in enough patients,” Powles said. “It’s sort of that Goldilocks effect of not too late and not too early, which is why I think that the [JAVELIN Bladder 100 trial (NCT02603432)] turned out to be a success.”
The phase 3 trial served as the rationale for the approval of avelumab in the frontline maintenance setting after the agent resulted in an improvement in overall survival vs best supportive care in both the overall cohort (HR, 0.69; 95% CI, 0.56-0.86; P = .001) and patients with PD-L1–positive tumors (HR, 0.56; 95% CI, 0.40-.079; P <.001).8,9
“In general, I offer maintenance avelumab to all the patients who satisfy the criteria in the trial. That includes patients with complete response, partial response, or stable disease with 4 to 6 cycles of platinum-based chemotherapy,” said Sonpavde. He went on to say that he considers holding maintenance only for certain patients—those with lymph node–positive disease, in whom he is confident of complete remission, out of concern of overtreatment.
“The vast majority of patients are progressing. Only [about] one-fifth of these patients are ever going to receive second-line therapy because they [progress] so fast,” Agarwal said. “Based on those 2 reasons, I don’t have any reason to wait for disease progression to happen before I can talk about immunotherapy. For me, this is a fantastic opportunity for our patients to receive an immunotherapy in the first line without having to wait for disease progression.1”
Patient Case Discussion
Grivas then brought the conversation to its practical application in a patient case.
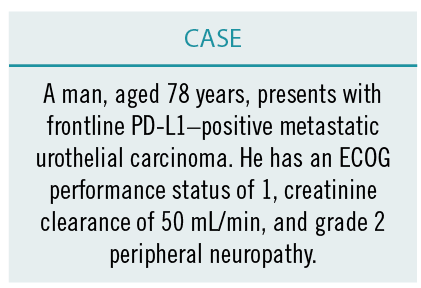
“This patient has a creatinine clearance of 50 mL/min. Sonpavde and colleagues had published data on splitting the cisplatin between the creatinine clearance of 50 and 60 mL/min. But based on peripheral neuropathy, I would not be inclined to do that. In this case, not only is kidney function impaired but the patient also has neuropathy, and that brings him into the realm of cisplatin ineligibility. It makes it more clear to me, so I would definitely offer carboplatin rather than an ICI alone,” said Agarwal.
“I agree that the JAVELIN paradigm of carboplatin and gemcitabine for 4 to 6 cycles followed by switch maintenance avelumab is reasonable, but I think we should remember pembrolizumab. The higher level of evidence would favor the JAVELIN paradigm because it’s a phase 3 trial, but I think that first-line pembrolizumab or atezolizumab is reasonable in this patient,” Sonpavde said.
When choosing chemotherapy followed by maintenance immunotherapy in a patient such as this, Powles did consider the toxicity profile of this regimen. “Not everyone has that easy journey, and many patients are beginning to struggle a little bit by the time they get to cycle 4, 5, or 6. Then there comes a question about how hard you want to push the toxicity, whether you want a dose reduction, or if you want to give all 6 cycles…[For] many patients, if they’ve had a rough journey with chemotherapy and want a few weeks off, I understand that.”
Despite a patient’s potentially difficult journey through cycles of chemotherapy, Powles concluded by highlighting the overall tolerable safety profile of maintenance immunotherapy.
“[What] we do know is [that] the adverse [effect] profile of avelumab and the other ICIs, and quality-of-life data, do suggest that things would be relatively stable. On the whole, it’s reasonable to say to patients, ‘The second part of the journey’s probably a bit easier than the first part,’” he concluded.
EDITOR’S NOTE: Interview quotes slightly modified for readability.
References
1. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy.
J Clin Oncol.1999;17(10):3173-3181. doi:10.1200/JCO.1999.17.10.3173
2. Swami U, Grivas P, Pal SK, Agarwal N. Utilization of systemic therapy for treatment of advanced urothelial carcinoma: lessons from real world experience. Cancer Treat Res Commun. 2021;27:100325. doi:10.1016/j.ctarc.2021.100325
3. NCCN. Clinical Practice Guidelines in Oncology. Bladder cancer, version 4.2021. Accessed August 6, 2021. https://bit.ly/37oHkDb
4. Pembrolizumab (Keytruda): advanced or metastatic urothelial carcinoma. FDA. May 19, 2017. Accessed August 6, 2021. https://bit.ly/3yGt3h2
5. FDA grants Genentech’s Tecentriq (atezolizumab) accelerated approval as initial treatment for certain people with advanced bladder cancer. News release. Genentech; April 17, 2017. Accessed August 6, 2021. https://bwnews.pr/2VCk424
6. FDA limits the use of Tecentriq and Keytruda for some urothelial cancer patients. FDA. July 5, 2018. Accessed August 6, 2021. https://bit.ly/3iqcSyY
7. Powles T, Huddart RA, Elliot T, et al. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2–positive metastatic bladder cancer. J Clin Oncol. 2017;35(1):48-55. doi:10.1200/JCO.2015.66.3468
8. FDA approves avelumab for urothelial carcinoma maintenance treatment. FDA. June 30, 2020. Accessed August 6, 2021. https://bit.ly/3iqcv7y
9. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-1230. doi:10.1056/NEJMoa2002788
EP: 1.Overview of Advanced Urothelial Carcinoma
EP: 2.Diagnosis of Advanced Urothelial Carcinoma
EP: 3.Standard of Care for First-Line Advanced Urothelial Cancer
EP: 4.Carboplatin vs I/O in Cisplatin-Ineligible Urothelial Cancer
EP: 5.Role of Maintenance Therapy in Advanced Urothelial Carcinoma
EP: 6.JAVELIN Bladder 100 Study in Advanced Urothelial Cancer
EP: 7.Patient Case: 78-Year-Old Man With mUC
EP: 8.Using Avelumab Maintenance in Advanced Urothelial Cancer
EP: 9.Clinical Impact of Biomarkers on Advanced Urothelial Cancer
EP: 10.Adjuvant ICI Therapy in Advanced Urothelial Cancer
EP: 11.Metastases and Variant Histology in Urothelial Cancer
EP: 12.Advanced Urothelial Cancer: Updates in Frontline Systemic Therapy
Recap: Recent Advances in the Treatment of Metastatic Castration-Sensitive Prostate Cancer
September 18th 2022Expert oncologists review key studies in the metastatic castration-resistant prostate cancer treatment landscape and discuss how evidence can be applied to clinical practice to improve patient outcomes.
Recap: Updates in Treatment of HER2-Positive Breast Cancer and Brain Metastases
July 16th 2022Sara A. Hurvitz, MD; Stefania Maraka, MD; and Ruta Rao, MD, discuss the evolving landscape of metastatic HER2+ breast cancer, highlighting recent clinical trials and the management of patients with brain metastases.
Recap: Emory Experts Review Treatment Strategies for Transplant-Ineligible Multiple Myeloma
June 20th 2022A panel of experts from Emory University review several key data updates in multiple myeloma from recent meetings and discuss how the data can be applied to clinical practice to improve patient outcomes.
