Aggressive colon cancer treatment: A matter of tolerability vs necessity
New biologics are improving the treatment of advanced colorectal cancer, but their optimal integration into practice remains unclear, and the question of how aggressive treatment should be is still open ended.
New biologics are improving the treatment of advanced colorectal cancer, but their optimal integration into practice remains unclear, and the question of how aggressive treatment should be is still open ended.

“NCCN guidelines say that if the patient can tolerate aggressive treatment, then treat the patient aggressively. But I believe the key question is not tolerability but whether the patient really needs aggressive treatment,” said Alberto Sobrero, MD, of the Ospedale San Martino in Genoa, Italy, “We are not there just to provide drugs but to provide sound decisions.”
Aggressive treatment is appropriate for patients with resectable tumors, and for patients with minimal or nonresectable disease who push for an aggressive approach. Resectability essentially determines curability and therefore ranks first in consideration. In some cases, patient age and performance factors should also be considered. In nonresectable cases, the presence of symptoms, tumor volume (high or low), and predicted clinical course (slow or rapid) are determinants, he said (ESMO 2008).
Therefore, if resection is medically sensible, the next question is whether it is technically feasible, Dr. Sobrero stated. Initially, unresectable tumors may be rendered resectable with aggressive treatment; if this potential is lacking, then the need for aggressive treatment is based on tumor-related factors.
First-line aggressive and nonaggressive strategies involve different regimens and have different endpoints (see Table). Aggressive treatment should impact response rates and symptoms, the goal being to shrink the tumor, he said. Nonaggressive strategies aim to improve quality of life and extend progression-free survival (PFS). Aggressive treatment is delivered intensely but only for three to six months, followed by maintenance therapy. Nonaggressive strategies are more prolonged but less intense and incorporate drug holidays.
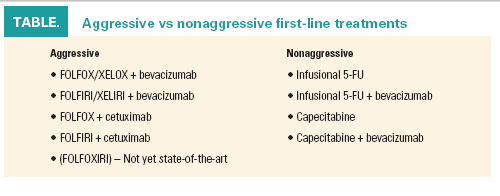
Doublets plus bevacizumab (Avastin) given first-line enhance the efficacy of any chemotherapy; such regimens have produced the longest PFS and overall survival (OS) outcomes to date, although response rates are increased only marginally. Benefit is obtained regardless of KRAS status; however, an unplanned analysis of the AVF2107 study of bevacizumab plus IFL showed benefit to be greater in wild-type patients, Dr. Sobrero noted (Herbert I. Hurwitz, MD, World Congress on Gastrointestinal Cancer 2008; Oncologist 14:22-28, 2009).
With doublets plus cetuximab (Erbitux), gain is harder to show because of the dependence on KRAS status. These regimens produce marginal benefit in PFS and no improvement in OS, although they consistently enhance response.
In the first-line CRYSTAL trial, the addition of cetuximab yielded a PFS gain of only 1.9 months in spite of an absolute 16% improvement in response (ASCO 2008 abstract 2). In the third-line, however, the addition of cetuximab to chemotherapy is more impressive, according to Dr. Sobrero.

The addition of cetuximab, unlike bevacizumab, also substantially increases toxicity, he added. Therefore, bevacizumab appears to more effectively delay progression whether patients have wild-type or mutated KRAS, and for tumor shrinkage is as effective as cetuximab in wild-type patients and more effective in patients with mutations. For these reasons, for patients who warrant aggressive treatment for advanced disease, regardless of KRAS status, Dr. Sobrero advocated first-line treatment with a chemotherapy doublet plus bevacizumab; a doublet plus cetuximab is acceptable in patients with wild-type KRAS.
For patients needing a nonaggressive approach, treatment with 5-FU or capecitabine (Xeloda) with or without bevacizumab is recommended. For salvage therapy, irinotecan plus oxaliplatin (Eloxatin), also known as IROX, has been shown to improve time to progression by 2.5 months and overall survival by 2.3 months, over irinotecan alone, he said (J Clin Oncol 26:4544-4550, 2008).
“For a nonaggressive approach, a single agent plus bevacizumab, and upon progression IROX plus or minus bevacizumab, can be a good approach,” Dr. Sobrero said. “The efficacy of cetuximab and panitumumab (Vectibix) is really outstanding in KRAS tumors.”