From anthracyclines to anti-VEGF: Shifts in the adjuvant treatment of breast cancer
ORLANDO-Breast cancer experts gathered at ASCO 2009 to answer five important questions about anthracyclines, taxanes, HER2-positive disease, trastuzumab (Herceptin), and anti-VEGF agents. Their key message for adjuvant therapy: One size does not fit all. The participants were:
ORLANDO-Breast cancer experts gathered at ASCO 2009 to answer five important questions about anthracyclines, taxanes, HER2-positive disease, trastuzumab (Herceptin), and anti-VEGF agents. Their key message for adjuvant therapy: One size does not fit all. The participants were:

• Clifford Hudis, MD, chief of the breast cancer medicine service at Memorial Sloan-Kettering Cancer Center, New York.
• Martine Piccart-Gebhart, MD, PhD, director of the department of medicine at Jules Bordet Institute, Brussels, and EORTC president.
• Sandra Swain, MD, medical director of the Washington Cancer Institute, Washington, DC.
1. Are anthracyclines always necessary?
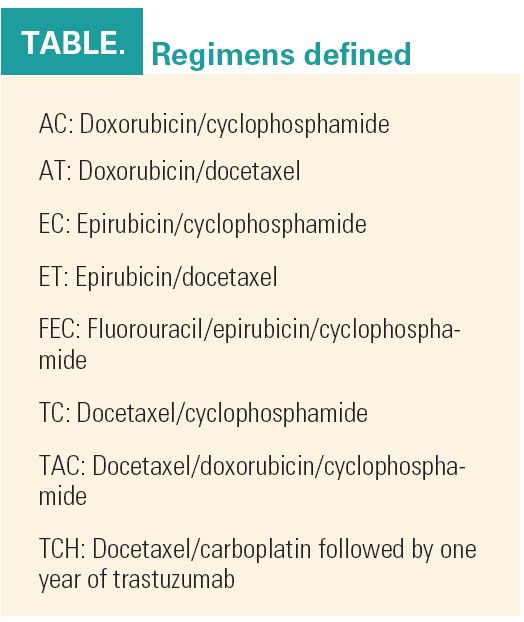
“Unquestionably, anthracyclines are inexpensive and effective in unselected patients, producing almost a 5% improvement in overall survival, but they have unique, serious, and potentially late toxicities,” Dr. Hudis said. “Can we avoid using anthracyclines? Should we phase them out entirely or safely replace them in certain cohorts?” (see Table on page 22). The answer is not a simple one because evidence for efficacy is observed in all subgroups. However, at least one recent study found nonanthracycline-based regimens to be as beneficial as anthracycline-based regimens.

In the seven-year follow up of US Oncology 9735 involving more than 1,000 patients, TC was superior to AC in disease-free survival (DFS) and overall survival (OS), Dr. Hudis noted (J Clin Oncol 27:1177-1183, 2009). But Japanese investigators confirmed that AC followed by a taxane was superior to a taxane alone (ASCO 2009 abstract 516).
In addition, a recent presentation by the NSABP of B-30 demonstrated that eight cycles of sequential AC followed by a taxane was superior to four cycles of TAC or AT. This suggests that the full doses of the components as afforded by sequential therapy improves outcomes and indirectly argues that a four cycle regimen like TC would be inferior to a standard sequence of anthracycline and taxane. “This keeps alive the concept that anthracyclines are critical components of adjuvant treatment,” Dr. Hudis said.
With regard to subgroups, can HER2 and topoisomerase II alpha (TOP2A) status guide treatment? Some investigators have found greater benefits in HER2-positive patients but a recent meta-analysis found that HER2 status was not a significant factor in OS (SABCS 2008 abstracts 6036 and 705).
The BCIRG 006 and MA5 trials suggested that anthracyclines may be unnecessary in patients who lack co-amplification of both HER2 and TOP2A, while those with TOP2A alterations (amplification or deletion) gain advantage by adding the anthracycline (SABCS 2006 abstract 52; J Natl Cancer Inst 101:644-650, 2009).
“New technologies are allowing us to drill further down on this question to clarify the genes of interest. Recent studies suggest there may be other genes on the TOP2A amplicon that can help predict benefit from anthracyclines,” he added.
Meanwhile, Dr. Hudis said clinicians should understand that such studies should not change practice. “We still do not have enough prospective, reproducible data to clearly show that differential benefit is lacking in any subset of patients. Most studies evaluating differential effectiveness in subsets have been retrospective, and they don’t all come to the same conclusion.”
Several important studies are evaluating the necessity of adjuvant anthracyclines, such as the TIC-TAC-TOE study and CALGB 40101, which is comparing dose-dense AC to paclitaxel for four cycles.
2. Who benefits from taxane therapy?
The 2007 Oxford Overview determined that there is an absolute 5% reduction in 10-year breast-cancer-specific mortality with the addition of taxanes to anthracycline-based regimens, according to Dr. Piccart-Gebhart. “But we are puzzled by the negative results in DFS of eight well-documented trials, including the very large (n = 4,000) TACT study from the United Kingdom,” she said (Lancet 373:1681-1692, 2009).
She cited 13 trials with positive results for taxanes (HR 0.58-0.86) and eight negative trials (HR 0.82-1.49). The mixed results may have been due to a lack of statistical power to detect clinically meaningful benefits, a particularly robust control arm, or a suboptimal dose or schedule of the taxane, she said, but when the studies are segregated according to these potential factors, no clear patterns emerge.
“In my view, the most likely explanation is contamination with low proliferative or luminal A disease,” she said. These tumors have a favorable prognosis, are associated with low OncotypeDX recurrence scores, and tend to show relative chemotherapy resistance. Study populations that include a high proportion of these molecular subtypes may fail to show benefits from chemotherapy, she explained.
This has been borne out by studies such as SWOG 8814, which showed a benefit from the addition of chemotherapy to tamoxifen only in patients with high recurrence scores (SABCS 2007 abstract LBA1005).
“We have learned that the benefit of taxane regimens is likely in highly proliferative tumors, which includes luminal B, triple-negative, and HER2-positive cancers,” she said.
In the future, gene expression patterns should reveal markers of taxane sensitivity that will be clinically useful. For example, when the Tau protein, which stabilizes microtubules, is downregulated, sensitivity to paclitaxel is increased. The BIG 01-EORTC 10994 trial will be evaluating such a multigene signature.
3. How do we optimize the adjuvant treatment of HER2-positive disease?
The value of trastuzumab in treating tumors that overexpress the HER2 protein was established years ago, but there are still questions about its optimal use. Numerous trials proved the value of giving trastuzumab after an anthracycline-taxane regimen, but BCIRG 006 found trastuzumab to be beneficial as part of a nonanthracycline regimen (TCH) as well, Dr. Swain pointed out.
The TCH regimen is now one of the preferred trastuzumab-containing options, along with AC followed by paclitaxel plus concurrent trastuzumab. Other acceptable regimens are docetaxel (Taxotere) plus trastuzumab followed by FEC; chemotherapy followed by trastuzumab (used in the HERA study); and AC followed by docetaxel plus trastuzumab, Dr. Swain said.
“All of these, with the exception of docetaxel used first, have been shown in large randomized controlled trials to be effective,” she said.
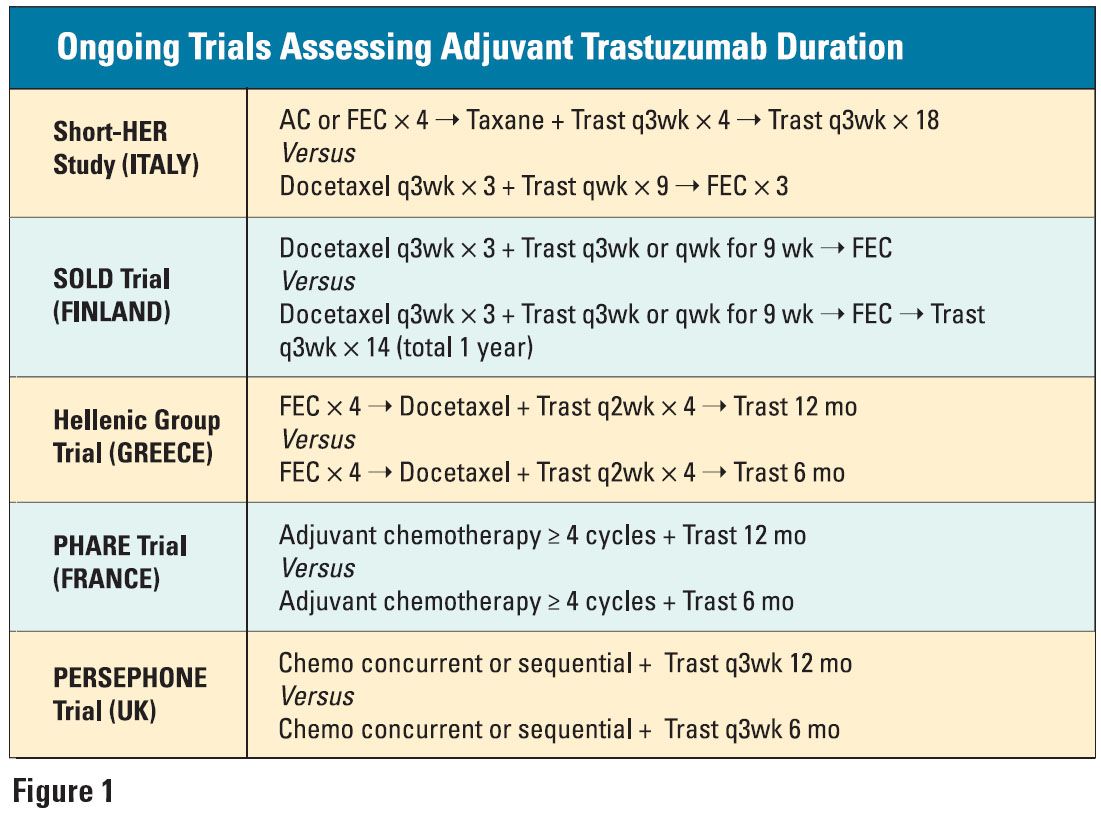
Still under debate is the optimal duration of trastuzumab treatment. While one year has been “arbitrarily adopted” as the standard, the HERA study is evaluating the benefit of giving trastuzumab for two years, and several European trials are also assessing various treatment durations (see Figure 1 on page 22).

Also unsettled is whether trastuzumab should be given sequentially after chemotherapy or concomitantly. Preclinical data suggest that the concomitant use is synergistic and cytotoxic and, therefore, preferred over the sequential delivery of the two components, which is only cytostatic.
But the clinical data remain confusing on this point, Dr. Swain added. In the HERA study, control patients who crossed over to receive trastuzumab had longer DFS that those who did not, “suggesting that even if you delay HER2-targeted therapy, you may still get a benefit,” she noted. However, a preliminary subanalysis of NCCTG N9831 found improvements in DFS and OS only with concurrent, not sequential, trastuzumab, and sequential trastuzumab was also ineffective following FEC or ET in the PACS 04 trial (SABCS 2007 abstract 72).
At this point, the data support concomitant or sequential trastuzumab plus chemotherapy, Dr. Swain said.
4. What about trastuzumab in small tumors?
Node-negative patients with HER2-positive tumors ≤ 1 cm have a worse prognosis than those with small HER2-negative tumors. “One of the questions I am asked most often is whether to treat these women with trastuzumab,” Dr. Swain said.
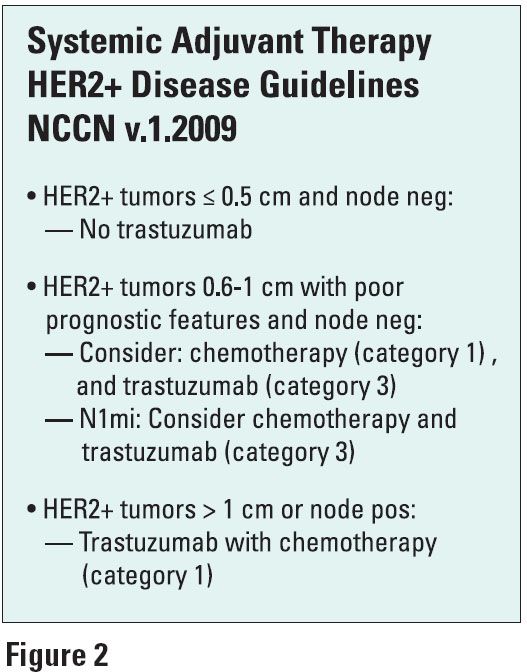
Her answer is typically yes, if the tumor ranges from 0.6 to 1 cm and depending on its other features. Based on the HERA and BCIRG trials, node-negative HERA patients with tumors of 1-2 cm had their risk reduced by 41% with trastuzumab (Ann Oncol 19:1090, 2008). New 2009 NCCN guidelines for HER2-positive disease are based on tumor size (see Figure 2).
The development of trastuzumab resistance is an emerging problem, and adjuvant tyrosine kinase inhibitors such as lapatinib (Tykerb) appear to be not only effective in this scenario but also less cardiotoxic, Dr. Swain said. A better understanding of the potential mechanisms of resistance will help in future drug designs.
5. What will be the role of anti-VEGF agents?
The VEGF is upregulated in HER2-positive breast cancer so there is a strong rationale for incorporating anti-VEGF agents, Dr. Swain said. In the metastatic setting, the combination of bevacizumab (Avastin) plus paclitaxel nearly doubled PFS in ECOG E2100. In the AVADO trial, bevacizumab plus docetaxel reduced progression by 39% (P = .0001). In addition, early-phase studies are showing high response rates when bevacizumab is combined with HER2-targeting agents (N Engl J Med 357:2666-2676, 2007; ASCO 2008 abstract LBA1011).
Bevacizumab, given in conjunction with adjuvant chemotherapy, adjuvant trastuzumab, and a taxane in the neoadjuvant setting, is currently under evaluation in a number of trials (see Related Reading on page 22 for details).
“The main message with all these components of adjuvant chemotherapy is that one size does not fit all, and future trials will have to take this into consideration,” Dr. Swain said.