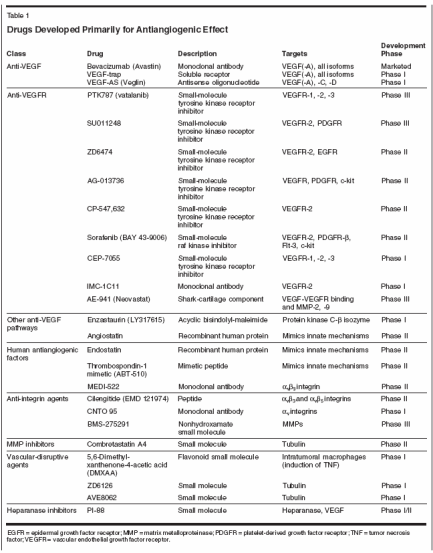
Antiangiogenic Agents in Cancer Therapy
There is substantial preclinical and clinical evidence that angiogenesisplays a role in the development of tumors and the progression ofmalignancies. Inhibiting angiogenesis has been shown to suppress tumorgrowth and metastasis in many preclinical models. These benefitshave translated to the clinic with both marketed and investigationalantiangiogenesis agents. The most prominent target of these compoundsis vascular endothelial growth factor (VEGF) and its receptors. However,several other factors are of interest as well. These include integrins,matrix metalloproteinases, and endogenous antiangiogenic factors. Datafrom late-stage clinical trials support the role of antiangiogenic agents incancer therapy and the significant role that VEGF plays in angiogenesis.Future research will focus on determining the tumor types and stages thatwill benefit most from antiangiogenic therapy and combining therapiesthat target different factors in the angiogenesis pathway.
There is substantial preclinical and clinical evidence that angiogenesis plays a role in the development of tumors and the progression of malignancies. Inhibiting angiogenesis has been shown to suppress tumor growth and metastasis in many preclinical models. These benefits have translated to the clinic with both marketed and investigational antiangiogenesis agents. The most prominent target of these compounds is vascular endothelial growth factor (VEGF) and its receptors. However, several other factors are of interest as well. These include integrins, matrix metalloproteinases, and endogenous antiangiogenic factors. Data from late-stage clinical trials support the role of antiangiogenic agents in cancer therapy and the significant role that VEGF plays in angiogenesis. Future research will focus on determining the tumor types and stages that will benefit most from antiangiogenic therapy and combining therapies that target different factors in the angiogenesis pathway.
There is substantial preclinical and clinical evidence that angiogenesis plays a role in the development of tumors and the progression of malignancies. As reviewed by Rakesh Jain in this supplement (see page 7), new tumors cease to grow beyond 1 to 3 cm without an additional blood supply. Once the angiogenic switch has occurred, the formation of new vessels begins, feeding the developing tumor and making it possible for it to progress. The number of new vessels formed, termed microvessel density, correlates with prognosis in many tumor types. This increased blood supply might be expected to aid in the delivery of chemotherapy to the tumor, but such is not the case. The tumor vasculature is irregularly shaped, disorganized, and highly permeable. This permeability contributes to high interstitial fluid pressure within the tumor, which inhibits the effective delivery of chemotherapy to the tumor tissue. The inefficient blood flow associated with this dysfunctional vasculature further impedes the delivery of chemotherapy. In addition, this dysfunctional vasculature can hinder tumor oxygenation, thereby promoting resistance to radiation. These effects of angiogenesis make this abnormal process in tumor development an attractive target for therapeutic intervention. A large number of endogenous pro- and antiangiogenic factors tightly regulate the process. Of these, the most prominent and well researched is vascular endothelial growth factor (VEGF). A phase III trial of the currently marketed monoclonal antibody to VEGF-A (referred to as VEGF), bevacizumab (Avastin), added to conventional cytotoxic chemotherapy showed greater survival in patients with metastatic colorectal cancer.[ 1] Many other agents that target VEGF and other factors in the regulation of angiogenesis are being developed. This article reviews agents currently marketed or in clinical development that target these factors. Current Antiangiogenic Therapies A large number of agents that target angiogenesis are in clinical development. They can be broadly classified as agents that have been developed primarily for their antiangiogenic activity (Table 1), and those that have been developed or used for other biologic effects but also have antiangiogenic activity (Table 2).[2] This review focuses on those that have been developed primarily as antiangiogenic agents.
VEGF-Targeted Agents
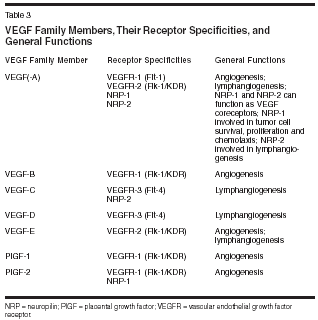
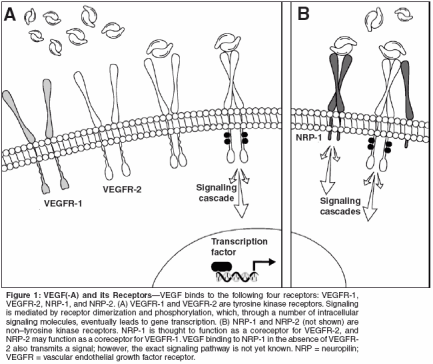
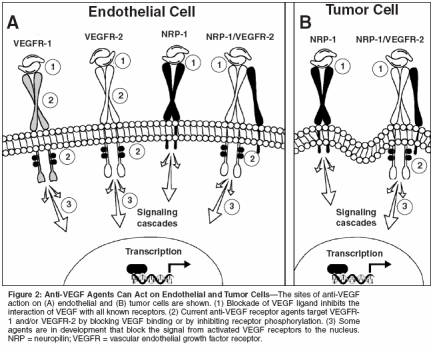
By far the greatest number of antiangiogenic drugs currently in clinical development target the VEGF pathway. As reviewed by Jain in this supplement, VEGF is crucial in the angiogenic process, making this pathway a logical target. VEGF belongs to a family of seven factors (Table 3). VEGF and its isoforms are the ligands for VEGF receptors (VEGFR)-1 and -2. Although VEGF binds to two receptor tyrosine kinases, VEGFR-1 (also known as Flt-1) and VEGFR-2 (also known as KDR), the angiogenic effects are primarily exerted through binding to VEGFR-2.[3] The binding of VEGF to these receptors on endothelial cells results in receptor dimerization and activation, which stimulates signaling cascades involving phospholipase C, protein kinase C, the Src tyrosine kinases, MAP kinase, phosphatidylinositol-3-kinase (PI3K), Ras GTPase-activating protein, and the Raf-Mek-Erk pathway (reviewed in [4,5]) (Figure 1). Additionally, VEGF binds to the non- tyrosine kinase neuropilin receptors (NRP-1 and NRP-2). NRP-1 can act as a coreceptor for VEGF, and as such potentiates VEGFR-2-dependent endothelial cell mitogenesis. Recently it has also been determined that NRP-1 regulates endothelial cell adhesion to extracellular matrix proteins, independent of VEGFR-2.[6] NRP-1 is also expressed on tumor cells and often is the sole VEGF receptor on these cells. Binding of VEGF to NRP-1 on tumor cells promotes tumor cell survival,[7] proliferation,[ 8] and chemotaxis.[9] The function of NRP-2 is as yet unclear but it is likely involved in lymphangiogenesis. Agents targeted toward VEGF act by antagonizing VEGF itself, blocking its receptors, inhibiting its synthesis, or inhibiting proteins in the VEGF receptor-signaling cascade. Most of these actions are targeted toward vascular endothelial cells; however, direct effects on tumor cells are possible through inhibiting interaction of VEGF with NRP-1 and with VEGFR-2 in the isolated cases in which this receptor is expressed on tumor cells (Figure 2). As reviewed by Jain in this supplement, there are two key mechanisms by which the antagonism of VEGF suppresses tumor growth and inhibits metastasis: prevention of neovascularization[10,11] and improvement of chemotherapy delivery.[ 12]
Despite the promising results with these agents, some in this class have failed in clinical trials due to poor efficacy and safety. Semaxinib (SU5416), which was active against VEGFR-1, -2, PDGFR, Flt-4, and c-kit, was associated with a high incidence of thrombotic events[27] and showed no increase in 1-year eventfree survival in a phase II trial in patients with renal cell carcinoma.[28] Similarly, no recent data have been reported on a related molecule, SU6668, which is active against VEGF, PDGF, and fibroblast growth factor-1 receptors. The reason for the delay or discontinuation of the development of SU6668 is not clear, but phase I trials showed self-inducing clearance, which resulted in difficulty in maintaining pharmacologically active concentrations with the maximum tolerated dose.[29-31] Other VEGF receptor-targeted agents include a raf kinase inhibitor, sorafenib (BAY 43-9006), and a chimeric monoclonal antibody to VEGFR-2, IMC-1C11, with sorafenib being the furthest along in development. In a phase II trial there was a response to sorafenib in 25 (40%) of 63 assessable patients with metastatic renal cell carcinoma.[32] The most common grade 3 adverse events were hand-foot skin reaction, rash, fatigue, and hypertension.[32] Good tolerability was shown with IMC-1C11 in phase I trials, but human antichimeric antibodies developed in half of the patients, and a second-generation human monoclonal antibody is being developed.[33]
- Other VEGF-Targeted Agents- Other agents that act on the VEGF pathway include AE-941 (Neovastat) and enzastaurin (LY317615). AE-941 is a derivative of shark cartilage that prevents VEGF from binding to its receptors and also inhibits matrix metalloproteinase (MMP) activity. AE-941 did not meet its primary end point, longer median overall survival, in a phase III trial in patients with renal cell carcinoma, but significantly longer survival was seen in the subgroup of healthier patients with clear cell histology and only one metastatic site (26.3 months with AE-941 vs 12.6 months with placebo; P = .02).[34] The agent is also very well tolerated.[35,36] Its development continues, and a phase III trial in NSCLC is ongoing.
Enzastaurin is an inhibitor of the protein kinase C-β isozyme, which is activated by VEGF and potentiates the effects of VEGF.[37] It is currently in phase I trials.[38] Agents That Do Not Target the VEGF Pathway
Several investigational antiangiogenic drugs are aimed at targets other than the VEGF pathway, including endogenous antiangiogenic factors, integrin inhibitors, enzyme inhibitors, and vascular-disruptive agents. None of these, however, have yet advanced to phase III clinical trials. Those in phase II trials are discussed here.
- Antiangiogenic Factors- Proteins that mimic naturally occurring antiangiogenic factors have been developed. Those on which phase II data are available include recombinant human angiostatin and endostatin. Preliminary phase II data on angiostatin in combination with paclitaxel and carboplatin in patients with NSCLC show partial responses in 39% and stable disease in 39% of 23 patients.[ 39] The duration of response was 8 months or longer in four of these patients. The most common adverse event was rash, in 92% of patients (grade 1 in 75%). There were few adverse events in phase I trials of angiostatin monotherapy, mainly grade 1 or 2 injection-site reactions.[ 40,41] Hemorrhage in central nervous system metastases occurred in two patients, and deep-vein thrombosis in two.[41] Long-term treatment (>1 year in five patients) resulted in no additional treatment-related adverse events.[41] A phase II study of endostatin in patients with advanced neuroendocrine tumors showed stable disease in 62% of patients.[42] The only severe adverse event was myocardial infarction in a patient with prior coronary artery disease.
- Integrin-Targeted Agents- Several molecules have been designed to target αv integrins. Integrins are heterodimeric cell-surface receptors that play an important role in endothelial cell migration, proliferation, and survival.[ 43] Two molecules are in phase II trials: MEDI-522 (Vitaxin) and cilengitide (EMD 121974). MEDI- 522 is a humanized monoclonal antibody to αvβ3.[44] Phase I trials showed an absence of serious adverse events other than infusion-related reactions.[ 44,45] Cilengitide is a peptide that contains the Arg-Gly-Asp (RGD) sequence. This sequence is present on integrins and is a binding epitope that is shared by many integrin ligands. Thus, RGD-containing peptides are thought to antagonize integrin-mediated actions.[43] Phase I trials showed no dose-limiting toxicities.[43,46] Phase II trials of both MEDI-522 and cilengitide are ongoing.
- Other Agents- A number of agents aimed at other targets have also been developed. These include the MMP inhibitor BMS-275291, the heparanase inhibitor PI-88, and a group of agents referred to as vascular- disruptive agents that attack existing tumor vasculature rather than prevent the formation of new vessels. BMS-275291 is a nonhydroxamate MMP inhibitor that has been designed to inhibit only MMPs that are not sheddases. It is thought that the inhibition of sheddases contributes to the arthrotoxicity associated with older hydroxamate MMP inhibitors.[47] Grade 2 or higher musculoskeletal symptoms developed in approximately a third of the patients in two phase II trials.[48,49] The drug was administered in both trials with paclitaxel, which is well known for causing arthralgias; however, the trials were randomized and the trial in breast cancer was halted early because of greater toxicity in the BMS-275291 group.[49] The trial in NSCLC, on the other hand, found no difference in arthrotoxicity between those receiving BMS-275291 and those receiving placebo (both along with paclitaxel and carboplatin), and patient accrual to a phase III trial is ongoing.[48] PI-88 inhibits heparanase, an enzyme that causes the release of proangiogenic factors from the extracellular matrix.[50] Good tolerability and evidence of efficacy with PI-88 in phase I trials has led to continued study.[50,51] A phase II trial in multiple myeloma showed disease stabilization in 39% of patients.[51] Phase II trials in melanoma, liver cancer, and NSCLC are ongoing.[51] Four small-molecule vascular-disruptive agents are in clinical trials.[52] Three of these, combretastatin A4, ZD6126, and AVE8062, cause the destruction of tumor vasculature through tubulin depolymerization of proliferating endothelial cells. The fourth, 5,6-dimethylxanthenone-4-acetic acid, is a flavonoid that induces the release of tumor necrosis factor from intratumoral macrophages. Combretastatin A4 is the furthest along in development, with phase II trials ongoing. The most common dose-limiting adverse event in phase I trials was tumor pain.[53,54] Others were acute coronary syndrome, ataxia, and motor neuropathy.
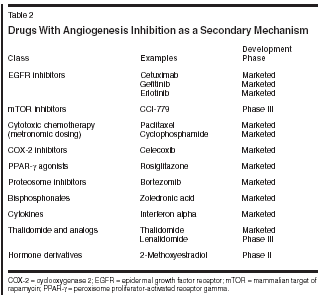
Agents With Secondary Antiangiogenic Activity
Agents that were developed primarily for other mechanisms but also have antiangiogenic activity are listed in Table 2. The most prominent of these agents are those that target EGFR, and several are currently marketed. Examples are the monoclonal antibody to HER2 trastuzumab (Herceptin) and the tyrosine kinase inhibitors gefitinib (Iressa) and erlotinib (Tarceva). Recent studies have shown that antiangiogenic activity contributes to the efficacy of this class of agents.[55,56] As reviewed by Folkman, ongoing research continues to elucidate the contributions of antiangiogenic mechanisms to the antitumor efficacy of many other marketed and investigational agents.[2] Therapeutic Considerations With Antiangiogenic Agents As more antiangiogenic agents become available, several issues must be considered, such as the potential for the development of resistance, when these agents should be used for optimal efficacy, and the differences among them. Classic mutational resistance mechanisms that plague traditional cytotoxic chemotherapy are less likely with antiangiogenic agents. Endothelial cells are more genetically stable than tumor cells, resulting in fewer mutations and less opportunity for the development of resistance.[57] However, as reviewed by Bergers and Benjamin, other mechanisms for resistance can occur.[57] Of particular interest is the role that p53 plays in sensitivity to the inhibition of angiogenesis. There is experimental evidence that the loss of p53 expression produces resistance to hypoxiainduced apoptosis.[58] Some preclinical studies suggest that combinations of antiangiogenic agents that have different mechanisms may be more effective than one agent alone, and this strategy may help prevent or overcome resistance.[57] Other factors that may affect the efficacy of antiangiogenics are the tumor stage and type. In mouse models of pancreatic islet cell carcinoma antiangiogenic agents with different mechanisms showed differential effects by tumor stage, with some preventing progression at an early stage and others causing the regression of more advanced tumors.[59] Thus, it will be important in clinical trials to assess when antiangiogenic agents should be used for optimal efficacy. The tumor type may also be important. A study of archival tumor samples found that the degree of angiogenesis differed significantly among tumor types.[60] This study also found that the maturity of the tumor vasculature might differ among tumors, as suggested by pericyte recruitment. Although two angiogenesis inhibitors similarly inhibited the growth of slow-growing, poorly vascularized tumors and of fast-growing, highly vascularized tumors in a murine model,[61] the differences among tumor types warrant thorough investigation in the clinic. Just as differences among tumors must be considered, so too must differences among angiogenesis inhibitors. Because this therapeutic modality is very new, clinically meaningful differences among agents are not known in the absence of direct comparisons of the agents. Certain differences should be considered, however, particularly among the large number of anti-VEGF pathway agents, as emerging data are evaluated. For example, bevacizumab prevents VEGF from binding to the neuropilin-1 receptor (NRP-1) and VEGFR tyrosine kinase inhibitors do not. The role of NRP-1 in tumor progression is still being investigated, but in vitro studies have shown that the binding of VEGF to NRP-1 augments angiogenesis,[62] prevents tumor cell apoptosis,[7] and promotes tumor cell proliferation.[8] Another consideration is pharmacokinetics. Small-molecule inhibitors such as the tyrosine kinase inhibitors may have shorter half-lives than monoclonal antibodies. For example, the average half-life of vatalanib is approximately 5 hours and that of bevacizumab is approximately 20 days.[13,63] It is clear that a substantial number of factors will play a role in the optimal use of angiogenesis inhibitors. This therapeutic field is in its infancy, and answers to these and other questions will be sought in new and ongoing clinical trials. Conclusions With one agent marketed and a large number of new products in development, the field of antiangiogenic therapy is growing rapidly. The relative lack of serious side effects compared to chemotherapy makes these agents an extremely attractive option. The survival advantage seen in the phase III trial of bevacizumab with irinotecan, 5-FU, and leucovorin in metastatic colorectal cancer estab- lishes the place of antiangiogenic agents in cancer therapy and shows the significant role that VEGF plays in angiogenesis. Future research with antiangiogenic therapies will focus on determining the tumor types and stages of cancer that will benefit from this modality, and on combining therapies that target different factors in the angiogenesis pathway.
Disclosures:
The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Eng J Med 350:2335-2342, 2004.
2. Folkman J: Angiogenesis inhibitors: A new class of drugs. Cancer Bio Ther 2(4 suppl 1):S127-S133, 2003.
3. Gille H, Kowalski J, Li B, et al: Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem 276:3222-30, 2001.
4. Gerwins P, Skoldenberg E, Claesson- Welsh L: Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Crit Rev Oncol Hematol 34:185-194, 2000.
5. Dvorak HF: Vascular permeability factor/ vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20:4368-4380, 2002.
6. Murga M, Fernandez-Capetillo O, Tosato G: Neuropilin-1 regulates attachment in human endothelial cells independently of vascular endothelial growth factor receptor-2. Blood 105:1992-1999, 2005.
7. Bachelder RE, Crago A, Chung J, et al: Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res 61:5736-5740, 2001.
8. Li M, Yang H, Chai H, et al: Pancreatic carcinoma cells express neuropilins and vascular endothelial growth factor, but not vascular endothelial growth factor receptors. Cancer 101:2341-2350, 2004.
9. Bachelder RE, Lipscomb EA, Lin X, et al: Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res 63:5230-5233, 2003.
10. Kim KJ, Li B, Winer J, et al: Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841-844, 1993.
11. Warren RS, Yuan H, Matli MR, et al: Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 95:1789-1797, 1995.
12. Wildiers H, Guetens G, De Boeck G, et al: Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer 88:1979-1986, 2003.
13. Avastin (bevacizumab) prescribing information. South San Francisco, Genentech, 2004.
14. Johnson DH, Fehrenbacher L, Novotny W, et al: Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22:2184-2191, 2004.
15. Miller KD, Chap LI, Holmes FA, et al: Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792-799, 2005.
16. Saltz LB, Lenz H, Kindler H, et al: Interim report of randomized phase II trial of cetuximab/bevacizumab/irinotecan (CBI) versus cetuximab/bevacizumab in irinotecanrefractory colorectal cancer (abstract 169b). Gastrointestinal Cancers Symposium, 2005.
17. Dickler M, Rugo H, Caravelli J, et al: Phase II trial of erlotinib (OSI-774), an epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor, and bevacizumab, a recombinant humanized monoclonal antibody to vascular endothelial growth factor (VEGF), in patients (pts) with metastatic breast cancer (MBC) (abstract 2001). Proc Am Soc Clin Oncol 23:127, 2004.
18. Hainsworth JD, Sosman JA, Spigel DR, et al: Phase II trial of bevacizumab and erlotinib in patients with metastatic renal carcinoma (RCC) (abstract 4502). Proc Am Soc Clin Oncol 23:381, 2004.
19. Mauer AM, Cohen EEW, Wong SJ, et al: Phase I study of epidermal growth factor receptor (EGFR) inhibitor, erlotinib, and vascular endothelial growth factor monoclonal antibody, bevacizumab, in recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) (abstract 5539). Proc Am Soc Clin Oncol 23:496, 2004.
20. Sandler AB, Blumenschein GR, Henderson T, et al: Phase I/II trial evaluating the anti-VEGF MAb bevacizumab in combination with erlotinib, a HER1/EGFR-TK inhibitor, for patients with recurrent non-small cell lung cancer (abstract 2000). Proc Am Soc Clin Oncol 23:127, 2004.
21. Dupont J, Schwartz L, Koutcher D, et al: Phase I and pharmacokinetic study of VEGF Trap administered subcutaneously (sc) to patients (pts) with advanced solid malignancies (abstract 3009). Proc Am Soc Clin Oncol 23:197, 2004.
22. Levine AM, Quinn DI, Gorospe G, et al: Phase I trial of anti-sense oligonucleotide vascular endothelial growth factor (VEGF-AS, Veglin) in patients with relapsed and refractory malignancies (abstract 3008). Proc Am Soc Clin Oncol 23:197, 2004.
23. Schleucher N, Trarbach T, Junker U, et al: Phase I/II study of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor in combination with FOLFIRI as first-line treatment for patients with metastatic colorectal cancer (abstract 3558). Proc Am Soc Clin Oncol 23:260, 2004.
24. Steward WP, Thomas A, Morgan B, et al: Expanded phase I/II study of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in combination with FOLFOX-4 as first-line treatment for patients with metastatic colorectal cancer (abstract 3556). Proc Am Soc Clin Oncol 23:259, 2004.
25. Rosen L, Mulay M, Long J, et al: Phase I trial of SU011248, a novel tyrosine kinase inhibitor in advanced solid tumors (abstract 765). Proc Am Soc Clin Oncol 22:191, 2003.
26. Motzer RJ, Rini BI, Michaelson MD, et al: SU011248, a novel tyrosine kinase inhibitor, shows antitumor activity in second-line therapy for patients with metastatic renal cell carcinoma: Results of a phase 2 trial (abstract 4500). Proc Am Soc Clin Oncol 23:381, 2004.
27. Kuenen BC, Rosen L, Smit EF, et al: Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J Clin Oncol 20:1657-1667, 2002.
28. Lara PN Jr, Quinn DI, Margolin K, et al: SU5416 plus interferon alpha in advanced renal cell carcinoma: A phase II California Cancer Consortium study with biological and imaging correlates of angiogenesis inhibition. Clin Cancer Res 9:4772-4781, 2003.
29. Brahmer JR, Kelsey S, Scigalla P, et al: A phase I study of SU6668 in patients with refractory solid tumors (abstract 335). Proc Am Soc Clin Oncol 21:84a, 2002.
30. Britten CD, Rosen L, Kabbinavar F, et al: Phase I trial of SU6668, a small molecule receptor tyrosine kinase inhibitor, given twice daily in patients with advanced cancers (abstract 1922). Proc Am Soc Clin Oncol 21:28b, 2002.
31. Kuenen B, Ruijter R, Hoekman K, et al: Dose finding study of SU6668 given thrice daily by oral route under fed conditions in patients with advanced malignancies (abstract 437). Proc Am Soc Clin Oncol 21:110a, 2002.
32. Ratain MJ, Flaherty KT, Stadler WM, et al: Preliminary antitumor activity of BAY 43- 9006 in metastatic renal cell carcinoma and other advanced refractory solid tumors in a phase II randomized discontinuation trial (RDT) (abstract 4501). Proc Am Soc Clin Oncol 23:381, 2004.
33. Posey JA, Ng TC, Yang B, et al: A phase I study of anti-kinase insert domain-containing receptor antibody, IMC-1C11, in patients with liver metastases from colorectal carcinoma. Clin Cancer Res 9:1323-1332, 2003.
34. Ãterna Zentaris: Ãterna Laboratories reports phase III trial results in renal cell carcinoma with Neovastat. Available at http:// www.aeternazentaris.com/en/page.php? p=60&q=46, 2003. Accessed March 1, 2005.
35. Batist G, Patenaude F, Champagne P, et al: Neovastat (AE-941) in refractory renal cell carcinoma patients: Report of a phase II trial with two dose levels. Ann Oncol 13:1259-1263, 2002.
36. Latreille J, Batist G, Laberge F, et al: Phase I/II trial of the safety and efficacy of AE-941 (Neovastat) in the treatment of non-small-cell lung cancer. Clin Lung Cancer 4:231-236, 2003.
37. Suzuma K, Takahara N, Suzuma I, et al: Characterization of protein kinase C beta isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc Natl Acad Sci U S A 99:721-726, 2002.
38. Rademaker-Lakhai JM, Beereport L, Witteveen EO, et al: Phase I and pharmacologic study of enzastaurin HCl, gemcitabine and cisplatin (abstract 3129). Proc Am Soc Clin Oncol 23:227, 2004.
39. Hanna NH, Estes D, Cress A, et al: Recombinant human angiostatin (rhAngiostatin) in combination with paclitaxel and carboplatin in patients (pts) with advanced NSCLC: Preliminary results of a phase II trial (abstract 7105). Proc Am Soc Clin Oncol 23:639, 2004.
40. DeMoraes ED, Fogler WE, Grant D, et al: Recombinant human angiostatin (rhA): A phase I clinical trial assessing safety, pharmacokinetics (PK) and pharmacodynamics (PD) (abstract 10). Proc Am Soc Clin Oncol 20:3a, 2001.
41. Beerepoot LV, Witteveen EO, Groenewegen G, et al: Recombinant human angiostatin by twice-daily subcutaneous injection in advanced cancer: A pharmacokinetic and long-term safety study. Clin Cancer Res 9:4025-4033, 2003.
42. Kulke M, Bergsland E, Ryan DP, et al: A phase II, open-label, safety, pharmacokinetic, and efficacy study of recombinant human endostatin in patients with advanced neuroendocrine tumors (abstract 958). Proc Am Soc Clin Oncol 22:239, 2003.
43. Holden SN, Morrow M, O’Bryant C, et al: Correlative biological assays used to guide dose escalation in a phase I study of the antiangiogenic αVβ3 and αVβ5 integrin antagonist EMD 121974 (EMD) (abstract 110). Proc Am Soc Clin Oncol 21:28a, 2002.
44. Faivre SJ, Chieze S, Marty M, et al: Safety profile and pharmacokinetic analysis of medi-522, a novel humanized monoclonal antibody that targets αvβ3 integrin receptor, in patients with refractory solid tumors (abstract 832). Proc Am Soc Clin Oncol 22:208, 2003.
45. Pizzolato JF, Sharma S, Maki R, et al: Phase I study of Medi-522, an αVβ3 integrin inhibitor, in patients (pts) with irinotecan-refractory colorectal cancer (CRC) (abstract 983). Proc Am Soc Clin Oncol 22:245, 2003.
46. Eskens FA, Dumez H, Hoekstra R, et al: Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer 39:917-926, 2003.
47. Rizvi NA, Humphrey JS, Ness EA, et al: A phase I study of oral BMS-275291, a novel nonhydroxamate sheddase-sparing matrix metalloproteinase inhibitor, in patients with advanced or metastatic cancer. Clin Cancer Res 10:1963-1970, 2004.
48. Douillard JY, Peschel C, Shepherd F, et al: Randomized phase II feasibility study of combining the matrix metalloproteinase inhibitor BMS-275291 with paclitaxel plus carboplatin in advanced non-small cell lung cancer. Lung Cancer 46:361-368, 2004.
49. Miller KD, Saphner TJ, Waterhouse DM, et al: A randomized phase II feasibility trial of BMS-275291 in patients with early stage breast cancer. Clin Cancer Res 10:1971-1975, 2004.
50. Holden SN, Basche M, Gore L, et al: A phase I study of the heparanase inhibitor PI-88 and weekly docetaxel in patients (pts) with advanced solid malignancies (abstract 899). Proc Am Soc Clin Oncol, 2003.
51. Progen, Inc. PI-88 (2004). Available at http://www.progen.com.au/re/Progen_PI- 88_Non_Confidential_Summary_May_04.pdf. Accessed March 1, 2005.
52. Siemann DW, Chaplin DJ, Horsman MR: Vascular-targeting therapies for treatment of malignant disease. Cancer 100:2491-2499, 2004.
53. Dowlati A, Robertson K, Cooney M, et al: A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res 62:3408-3416, 2002.
54. Rustin GJ, Galbraith SM, Anderson H, et al: Phase I clinical trial of weekly combretastatin A4 phosphate: Clinical and pharmacokinetic results. J Clin Oncol 21:2815- 2822, 2003.
55. Klos KS, Zhou X, Lee S, et al: Combined trastuzumab and paclitaxel treatment better inhibits ErbB-2-mediated angiogenesis in breast carcinoma through a more effective inhibition of Akt than either treatment alone. Cancer 98:1377-1385, 2003.
56. Asakuma J, Sumitomo M, Asano T, et al: Modulation of tumor growth and tumor induced angiogenesis after epidermal growth factor receptor inhibition by ZD1839 in renal cell carcinoma. J Urol 171:897-902, 2004.
57. Bergers G, Benjamin LE: Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3:401-410, 2003.
58. Yu JL, Rak JW, Coomber BL, et al: Effect of p53 status on tumor response to antiangiogenic therapy. Science 295:1526- 1528, 2002.
59. Bergers G, Javaherian K, Lo KM, et al: Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 284:808-812, 1999.
60. Eberhard A, Kahlert S, Goede W, et al: Heterogeneity of angiogenesis and blood vessel maturation in human tumors: Implications for antiangiogenic tumor therapies. Cancer Res 60:1388-1393, 2000.
61. Beecken WD, Fernandez A, Joussen AM, et al: Effect of antiangiogenic therapy on slowly growing, poorly vascularized tumors in mice. J Natl Cancer Inst 93:382-387, 2001.
62. Oh H,Takagi H, Otani A, et al: Selective induction of neuropilin-1 by vascular endothelial growth factor (VEGF): A mechanism contributing to VEGF-induced angiogenesis. Proc Nat Acad Sci U S A 99:383-388, 2002.
63. Drevs J, Mross K, Medinger M, et al: Phase I dose-escalation and pharmacokinetic (PK) study of the VEGF inhibitor PTK787/ZK 222584 (PTK/ZK) in patients with liver metastases (abstract 1142). Proc Am Soc Clin Oncol 22:284, 2003.