Antibody-drug conjugate represents a new direction for treatment of HER2-positive breast cancer patients
A first-in-class HER2 antibody-drug conjugate may represent an effective new strategy in breast cancer therapy, according to phase I data
ASCOA first-in-class HER2 antibody-drug conjugate may represent an effective new strategy in breast cancer therapy, according to phase I data that attracted much attention at the 43rd Annual Meeting of the American Society of Clinical Oncology (abstract 1042).
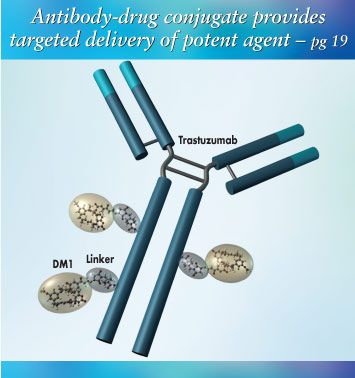
The investigational agent, trastuzumab-DM1 (T-DM1), is designed to combine the anti-tumor activity of trastuzumab with a means of delivering highly potent chemotherapy directly into HER2-expressing cells.
DM-1 (also known as maytansine) is an inhibitor of tubulin polymerization, binding tubulin competitively with vinca alkaloids. Compared with drugs like vincristine, however, it is 20 to 100 times more potent, said principal investigator Murali Beeram, MD, of the Institute for Drug Development, San Antonio.
"DM-1 was developed in 1975 but was found to be too toxic for standard chemotherapy," Dr. Beeram said. "We tagged it in a very small amount to the trastuzumab molecule with a linker. The linker provides a stable bond between the two agents that is designed to prolong exposure and reduce the toxicity of T-DM1. We are, in essence, sending a chemotherapy payload into the cell via the antibody. We get a twofold effectan increase in the efficacy of trastuzumab and direct delivery of the cytotoxic agent, which reduces treatment toxicity."
Dr. Beeram and colleagues presented data from 18 HER2-positive metastatic breast cancer patients who progressed after a trastuzumab-containing regimen. In addition to prior treatment with trastuzumab, these patients had received multiple prior chemotherapy regimens (median, 7.5).
Five dose levels of the immunoconjugate were evaluated: 0.3 to 4.8 mg/kg given intravenously every 3 weeks.
Of these heavily pretreated patients, four had a confirmed partial response with dosing at or below the MTD
(3.6 mg/kg), and the responses are ongoing. One patient has continued to respond for over a year, Dr. Beeram reported.
The compound was well tolerated. The only grade 3-4 toxicity was a rapidly reversible grade 4 thrombocytopenia at the highest dose in one patient. Cardiac toxicity has not been observed.
A phase II trial of the immunoconjugate is being initiated in HER2-positive metastatic breast cancer patients. Genentech, which is developing the new agent, has enlisted ImmunoGen, Inc. to develop a commercial-scale process for making
T-DM1, using ImmunoGen's tumor-activated prodrug (TAP) technology.
'Fascinating Concept'
Larry Norton, MD, physician-in-chief, Breast Cancer Program, Memorial Sloan-Kettering Cancer Center, commented that these findings provide encouragement for the development of immunoconjugates in breast cancer.
Dr. Norton told Oncology NEWS International: "This is a fascinating concept and encouraging data. The big issue with immunoconjugates is the problem of drug delivery. The method is very likely to make a treatment less toxic, but is it more effective? What is the therapeutic quality of the immunoconjugate?"
Dr. Norton said that while the abstract shows the possibility of delivering the drug, "the improvement in efficacy and therapeutic ratio, of course, needs to be determined. But this phase I study holds a lot of merit."
On the Cover
As shown in the cover image, the antibody-drug conjugate trastuzumab-DM1 (T-DM1) consists of ImmunoGen's potent cell-killing agent DM1 (derived from maytansine) attached by a chemical linker to Genentech's HER2-binding monoclonal antibody, trastuzumab (Herceptin). This creates a so-called armed antibody. T-DM1 is in development by Genentech, which provided the illustration.