Biphasic Tumors of the Female Genital Tract
In this installment of Second Opinion, we are presenting two cases of tumors of the female genital tract, specifically, the ovary and uterus, which contain both epithelial and mesenchymal components and therefore have unique diagnostic and therapeutic implications. The first has an unusually poor prognosis and the second is notoriously difficult to diagnose.
SECOND OPINION
Multidisciplinary Consultations on Challenging Cases
The University of Colorado Denver School of Medicine faculty holds weekly second opinion conferences focusing on cancer cases that represent most major cancer sites. Patients seen for second opinions are evaluated by an oncologic specialist. Their history, pathology, and radiographs are reviewed during the multidisciplinary conference, and then specific recommendations are made. These cases are usually challenging, and these conferences provide an outstanding educational opportunity for staff, fellows, and residents in training.
The second opinion conferences include actual cases from genitourinary, lung, melanoma, breast, neurosurgery, gastrointestinal, and medical oncology. On an occasional basis,
ONCOLOGY
will publish the more interesting case discussions and the resultant recommendations. We would appreciate your feedback; please contact us at
second.opinion@uchsc.edu
.
E. David Crawford, MD
Al Barqawi, MD
Guest Editors
University of Colorado Health Sciences Center
and Univeristy of Colorado Cancer Center
Denver, Colorado
In this installment of Second Opinion, we are presenting two cases of tumors of the female genital tract, specifically, the ovary and uterus, which contain both epithelial and mesenchymal components and therefore have unique diagnostic and therapeutic implications. The first has an unusually poor prognosis and the second is notoriously difficult to diagnose.
Patient 1
Clinical History
A 64-year-old nulliparous woman who had been postmenopausal for 12 years presented to the emergency room with vague right lower quadrant pain, early satiety, and constipation for 1 week. She denied anorexia, nausea or vomiting, and weight loss. Review of systems was otherwise negative. Gynecologic history was significant for the use of hormone-replacement therapy, which had been discontinued for 2 years, and a bilateral tubal ligation 6 years ago. A Pap smear, mammogram, and colonoscopy were up-to-date and reportedly normal. An abdominal computed tomography (CT) scan showed a 6-cm right lower quadrant mass, omental cake, and ascites. Pertinent blood work included an elevated CA-125 level of 329 microL/mL. She was referred to Dr. Davidson at University of Colorado Health Sciences Center (UCHSC) for a surgical consultation.
On physical examination, the patient's abdomen was notable for hypoactive bowel sounds, lower right quadrant tenderness, and a palpable fluid wave. Bimanual pelvic examination revealed a mass causing posterior uterine displacement. Posterior cul-de-sac nodularity was noted on rectal-vaginal examination. A repeat CT scan did not demonstrate periaortic adenopathy. These findings were consistent with ovarian cancer.
The patient underwent comprehensive staging. The surgical procedure consisted of total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO), rectosigmoid colon resection and primary anastomosis, appendectomy, omentectomy, and optimal tumor debulking.
Operative and Pathologic Findings
Dr. Ramona Evans: What were the operative findings?
Dr. Susan A. Davidson: Intraoperative exploration revealed a friable, nodular, pelvic mass arising from/attached to the right ovary. The tumor was adherent to the uterus, cecum, appendix, and distal ileum. Extensive tumor implants ranging in size from 0.1 to 5 cm were evident throughout the pelvic peritoneum, omentum, mesentery, distal ileum, cecum, appendix, transverse and sigmoid colon, and right side of the diaphragm. The left ovary was small but appeared to have surface disease. The stomach, spleen, pancreas, and porta hepatis were all normal in appearance, and enlarged lymph nodes were not identified.
Dr. Evans: What were the pathology findings? Was the perioperative diagnosis confirmed in the TAH/BSO specimen?
FIGURE 1
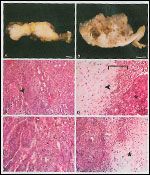
Tumor Findings in a 64-Year-Old Woman
Dr. Meenakshi Singh: A friable, solid, tan with translucent areas, 11-cm mass was arising from/attached to the right ovary (Figures 1A, 1B). Tumor was scattered through the residual parenchyma of the right ovary and was macroscopically evident on the serosal surfaces of the uterus, left adnexa, and peritoneum. The omental cake showed multiple tumor nodules of variable size.
Microscopic examination of the right ovarian/pelvic mass revealed a biphasic, high-grade, malignant neoplasm with carcinomatous epithelium and sarcomatous mesenchymal tissues (Figures 1C-F). The carcinoma displayed solid sheets, glands, and focal papillary architecture as well as squamous differentiation (Figure 1C). The sarcomatous component showed spindle cells with increased cellularity and frequent mitoses (Figure 1F). Focal heterologous elements, predominantly chondrosarcoma-like, were noted (Figures 1D, 1F). Areas of hemorrhage and necrosis were present throughout the tumor.
Additionally, the tumor involved the surface and parenchyma of the left ovary. Serosal tumor deposits were observed on the uterus, peritoneum, colon, and appendix. The omental cake was due to tumor. Metastatic tumor was present in five of five regional lymph nodes examined as well as in the pelvic wash fluid. The tumor was diagnosed as a carcinosarcoma (also known as malignant mixed mullerian tumor) and was staged as American Joint Committee on Cancer (AJCC) stage IIIC.
Diagnostic Considerations
Dr. Evans: What is the differential diagnosis for a tumor with this appearance?
Dr. Singh: A tumor with this histology is classic for carcinosarcoma. However, an additional biphasic tumor in this location and with infiltrative properties includes an ovarian endometrioid adenosarcoma (see discussion for patient 2).
Dr. Evans: What is the current thinking regarding the clonal origin of carcinosarcoma?
Dr. Singh: The majority of studies have actually been done on uterine carcinosarcomas, but the histology and pathogenesis of ovarian carcinosarcomas are thought to be similar. Immunohistochemical, ultrastructural, and molecular studies provide convincing evidence supporting a monoclonal origin for the majority of carcinosarcomas.[1] Ovarian carcinosarcomas are believed to arise from either ovarian surface epithelium or foci of endometriosis and can be regarded as endometrioid adenocarcinomas in which malignant stromal differentiation has occurred.[2] A small percentage of carcinosarcomas are not of monoclonal origin, but rather, represent true collision tumors.[1] One study suggests that 8% to 16% of carcinosarcomas actually arise within, or in the endometrial tissue adjacent to, an adenosarcoma.[3]
Dr. Evans: What are the clinical features of carcinosarcomas?
Dr. Davidson: Carcinosarcomas occur almost exclusively in postmenopausal women.[2,4,5] The clinical presentation of ovarian carcinosarcoma is similar to that of other ovarian carcinomas. Approximately 90% of ovarian carcinosarcomas are bilateral.[2] The tumor marker CA-125 is usually elevated.[4]
Dr. Evans: What is the epidemiology of carcinosarcoma?
Dr. Davidson: Carcinosarcomas of the ovary are very rare, constituting only 1% of all ovarian tumors.[5] Studies suggest a higher incidence of uterine carcinosarcomas in blacks.[6] Risk factors include age and low parity.[2,5] Exposure to radiation has long been considered to play a role in the development of carcinosarcoma.[2,4,6]
Treatment Options
Dr. Evans: What are the treatment options for patients diagnosed with carcinosarcoma?
Dr. Davidson: Surgery is the mainstay of treatment. A number of postoperative treatment modalities have been utilized, but a significantly effective adjuvant therapy regimen has yet to be established. A recent study of uterine carcinosarcoma suggests that combination chemotherapy followed by whole-pelvic irradiation after optimal tumor debulking surgery may incur a significant decrease in mortality when compared to postoperative chemotherapy alone.[6] Of the various chemotherapy regimens available, platinum-based combinations seem to be the most efficacious.[2,7] The overall response rate is reported at 20%, which is similar to that seen in the uterine counterpart.[7] Clinical studies under the auspices of the Gynecologic Oncology Group are under way and shall provide more information when their data are analyzed.
Dr. Evans: What is the prognosis for these tumors, how are they followed up, and what factors influence prognosis?
Dr. Davidson: The prognosis for carcinosarcoma of the ovary is dismal, with a median survival of only 19 months[2] and a 5-year survival rate of 18% to 27%.[2,5] The single most important prognostic indicator is the stage of tumor at the time of initial treatment,[5] which unfortunately is advanced for this patient. There are no other histopathologic features that significantly predict outcome.[5]
Patient 2
Clinical History
A 49-year-old woman (G2, P2), who had been postmenopausal for 10 years, presented to an outside hospital with abdominal pain. At that time, she underwent an emergent right salpingo-oophorectomy for a ruptured ovarian cyst. A CT scan revealed severe, right-sided hydronephrosis and an abnormal right kidney. Two subsequent pelvic examinations, 13 and 16 months later, revealed palpable vaginal masses. These were biopsied, and pathology was interpreted as fibroepithelial polypoid masses with lymphatic proliferation and numerous benign-appearing tubular epithelial glands without evidence of malignancy.
A rectal-pelvic exam performed 1 month after the second biopsy revealed a mass that had increased in size and extended posteriorly toward the rectum. A second CT scan and a magnetic resonance imaging (MRI) scan revealed a marked hydroureter and severe right-sided hydronephrosis with near-complete atrophy of the kidney. Two separate but adjacent masses in the right adnexal region and pouch of Douglas were also visualized. These findings were clinically worrisome for sarcoma. The patient underwent a posterior pelvic exenteration and partial colon resection with reanastomosis at the UCHSC.
Operative and Pathologic Findings
Dr. Elke Jarboe: What were the operative findings?
Dr. Kian Behbakht and Dr. Davidson: The surgery commenced with a right nephrectomy and freeing of the distal right ureter for the long-standing hydronephrosis and complete absence of kidney parenchyma. On palpation in the pelvis, a 4 x 5 cm firm mass was noted, involving the right parametrium, uterus, rectosigmoid colon at the level of the peritoneal reflection, and upper vagina. A radical dissection and posterior pelvic exenteration was required to encompass the entire lesion. A partial colectomy and primary anastomosis of the colon was then performed.
Dr. Dean Fong: What were the pathology findings? Was the perioperative diagnosis confirmed in the surgical specimen?
FIGURE 2

Tumor Findings in a 49-Year-Old Woman
Dr. Singh: The uterus, right ovary, right fallopian tube, distal right ureter, and portions of the colon and proximal vagina were received as one specimen. The right kidney, left adnexa (Figure 2A), and multiple lymph nodes were each received separately. A firm, 8-cm nodular mass was located within the right parametrium and paravaginal tissue (Figure 2B) and infiltrated the vaginal wall, colon, and ureter. Cut sections revealed a solid, whorled, tan surface. The right ureter revealed complete obstruction of the lumen by the tumor. The parametrial mass was contiguous with the uterine wall; however, sectioning of the uterus did not reveal a separate tumor mass (Figure 2B).
Microscopic examination revealed a widely infiltrative biphasic neoplasm (Figure 2C) with a cellular stroma surrounding cytologically benign, variably dilated endometrioid glands (Figure 2D). The stromal cell nuclei showed mild pleomorphism, and mitoses were not identified after a thorough examination (Figure 2E). This morphology is diagnostic of a low-grade adenosarcoma. The tumor protruded into the vagina as a nodule and invaded the colon from the serosa into the submucosa (Figure 2F). The ureter demonstrated luminal obstruction by the tumor (Figure 2G), and focal lymphovascular invasion was seen. The tumor was immunoreactive for estrogen receptor (ER) and progesterone receptor (PR), in both glands and stroma and for CD10 (endometrial stromal/mullerian tissue marker) in the stroma (Figure 2H). MIB-1 immunostain demonstrated a low proliferation index overall, although this was lower in the sarcomatous component as compared to the epithelial component (Figure 2I).
Bilateral ovaries and fallopian tubes, right kidney, and all nine lymph nodes were free of tumor. The right nephrectomy was remarkable for an atrophic, end-stage kidney with hydronephrosis and a hydroureter.
Diagnostic Considerations
Dr. Pamela Lyle: Are there other types of biphasic tumors in the female genital tract?
Dr. Singh: Tumors with mixed epithelial and mesenchymal elements most commonly occur in the uterus and include carcinosarcoma, atypical polypoid adenomyoma (APA), adenofibroma, and adenosarcoma. Besides APAs, the other aforementioned neoplasms can arise in the ovaries as well in foci of endometriosis. Furthermore, biphasic tumors are not limited to the genital tract. For example, in the breast, biphasic tumors include fibroadenoma, phylloides tumor, and metaplastic carcinoma.
Extraovarian carcinosarcomas have a histology and features similar to those described above for ovarian neoplasms. APAs are generally well-circumscribed, solitary, polypoid masses comprised of endometrioid glands with cytologic and architectural atypia and mitoses, intermixed within a myofibromatous stroma. Squamous morules are present in 90% of tumors.[4] The stromal component exhibits bundles of smooth muscle or myofibroblasts.
Adenofibromas are the benign counterpart of adenosarcomas. Although very similar histologically, the benign stromal component of an adenofibroma is mitotically inactive and lacks the nuclear atypia and periglandular cuffing exhibited by adenosarcomas (see below).[8,9] Adenosarcoma most commonly presents as a broad-based polypoidal mass within the endometrial cavity of the lower uterine segment. In this patient, the tumor did not form a polypoidal mass in the endometrial cavity and may have arisen in the parametrial or paravaginal tissues.
Adenosarcomas exhibit biphasic histology that generally consists of benign, often cystically dilated, glands admixed with a mesenchymal component, which usually consists of a low-grade sarcoma. The sarcomatous element of the tumor is typically most evident around the benign glands-a feature referred to as cuffing. These stromal cuffs tend to have greater cellularity, atypia, and stromal mitoses. If the mitoses exceed 2 to 4 per 10 high-powered fields, it helps confirm adenosarcoma when adenofibroma is being considered in the differential diagnosis.[8,9] Areas of adenofibroma may be evident within adenosarcomas.[8]
Dr. Fong: Why is it not one of the other previously mentioned entities?
Dr. Singh: Carcinosarcoma is essentially ruled out based on the absence of a frankly malignant glandular component. It is not an APA, as the tumor in this case lacks the glandular atypia, smooth muscle, and squamous morules that are generally demonstrated in APAs. Morphologically, the stroma of adenomafibroma lacks the hypercellularity and cuffing, which were focally evident in this case. The clinical presentation is also inconsistent with a benign process. The lack of mitoses in our case is unusual for adenosarcoma. It also had a low proliferative index (MIB-1 immunostain). However, the combination of clinical presentation and morphology confirms the diagnosis of adenosarcoma.
Dr. Lyle: What is the meaning of CD10 positivity in this case?
Dr. Singh: The tumor was positive for CD10 expression. CD10 was initially thought to be a marker for endometrial stromal sarcomas, but has since been shown to be positive in adenosarcomas and carcinosarcomas as well. It is now believed that it may be a marker for tissues of mullerian origin.[10,11].
Dr. Lyle: What is the frequency and behavior of adenosarcomas?
Dr. Singh: This is a rare tumor, which accounts for approximately 8% of all uterine sarcomas.[12] Adenosarcoma arising from vaginal endometriosis is a much rarer event.[8,13] Adenosarcomas have traditionally been regarded as a tumor of low-grade malignancy, behaving clinically like low-grade sarcomas with minimal propensity for distant metastasis.[8,9] However, cases of metastases to distant sites have been reported.[9] Recurrence of disease following surgery occurs in 25% to 40%[2] of patients and carries a bad prognosis.[2,8] Adenosarcomas originating outside of the endometrial cavity tend to present at a more advanced stage.[9]
Dr. Lyle: How does the patient's prior biopsy correlate with the findings in the excision specimen?
Dr. Singh: An earlier biopsy of the paravaginal mass from this patient revealed benign glands within fibromatous tissue and dilated lymphatic spaces (Figure 2J). It is not uncommon for extraovarian adenosarcomas to be diagnosed as benign polyps on small excision biopsies,[4,14] as these may not have all the components of adenosarcoma represented in them.
Dr. Lyle: What are the clinical features of adenosarcoma?
Dr. Davidson: Uterine adenosarcomas generally present in the 4th and 5th decades[2] as vaginal bleeding.[12] They often present as polypoidal lesions, frequently protruding through the external os.[12,14] Albeit rare, the possibility of adenosarcoma ought to be considered in the case of an enlarging mass at the site of extraovarian endometriosis.[8,13]
Dr. Jarboe: What risk factors are associated with adenosarcoma?
Dr. Singh: Some tumors have been associated with hyperestrogenic states, as evidenced by multiple case reports of tamoxifen-associated adenosarcomas.[15]
Treatment Options
Dr. Jarboe: What are the treatment options and prognosis for patients diagnosed with adenosarcoma?
Dr. Davidson: Surgery is the mainstay of treatment. Platin-based chemotherapy has also proven efficacious, particularly if given up front in inoperable patients. In select cases, the cautious use of aggressive therapy is warranted given the ominous clinical behavior of some adenosarcomas. Radiation therapy has also proven successful as an adjunctive treatment in certain cases.[8] In one series, the 5-year survival was 64%.[16]
Dr. Lyle: What treatment plan was selected for this case?
Dr. Behbakht: The patient's pathologic findings were discussed at the gynecologic oncology multidisciplinary conference. Given the extent of her disease, and presence of tumor at excision margins, it was determined that the benefits of radiotherapy outweighed the risk of radiation-induced transformation into a high-grade sarcoma. The consensus recommendation was treatment with whole-pelvic external-beam radiotherapy to minimize the chance of local recurrence.
Dr. Lyle: Does the ER/PR-positive status of this tumor affect therapy?
Dr. Behbakht: Since the tumor showed both the epithelial and stromal components to be strongly ER/PR-positive in a majority of the cells, postoperative hormonal therapy in the form of megestrol may improve survival. Amant et al[17] report that recurrent adenosarcoma is expected to respond to progesterone and gonadotropin-releasing hormone (GnRH) analogs. With a diagnosis of adenosarcoma, the woman should not be a candidate for hormone replacement therapy.
Financial Disclosure:The participants in this conference have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. McCluggage WG: Malignant biphasic uterine tumors: Carcinosarcomas or metaplastic carcinomas. J Clin Pathol 55:321-325, 2002.
2. Tavassoli FA, Devilee P: World Health Organization Classification of Tumours: Tumours of the Breast and Female Genital Organs, pp 133-134. Lyon, France, IARC publications, 2003.
3. Seidman JD, Chauhan S: Evaluation of the relationship between adenosarcoma and carcinosarcoma and a hypothesis of the histogenesis of uterine sarcomas. Int J Gynecol Pathol 22:75-82, 2003.
4. Clement PB, Young RH: Atlas of Gynecologic Surgical Pathology, pp 196-204. Philadelphia, W.B. Saunders, 2000.
5. Ariyoshi K, Kawauchi S, Kaku T, et al: Prognostic factors in ovarian carcinosarcoma: A clinicopathological and immunohistochemical analysis of 23 cases. Histopathology 37:427-436, 2000.
6. Menczer J, Levy T, Piura B, et al: A comparison between different postoperative treatment modalities of uterine carcinosarcoma. Gynecol Oncol 97:166-170, 2005.
7. Thigpen TJ, Blessing JA, DeGeest K, et al: Cisplatin as initial chemotherapy in ovarian carcinosarcomas: A Gynecologic Oncology Group study. Gynecol Oncol 93:336-339, 2004.
8. Lanting L, Davidson S, Singh M: Mullerian adenosarcoma of vagina arising in persistent endometriosis: Report of a case and review of the literature. Gynecol Oncol 90:486-490, 2003.
9. Gollard R, Kosty M, Bordin G, et al: Two unusual presentations of Mullerian adenosarcoma: Case reports, literature review, and treatment considerations. Gynecol Oncol 59:412-422, 1995.
10. Mikami Y, Hata S, Kiyokawa T, et al: Expression of CD10 in malignant mullerian mixed tumors and adenosarcomas: An immunohistochemical study. Mod Pathol 15:923-930, 2001.
11. Mikami Y: Reply to Letter to Editor. Mod Pathol 16:618-619, 2003.
12. Piura B, Rabinovich A, Meirovitz M, et al: Mullerian adenosarcoma of the uterus: Case report and review of literature. Eur J Gynecol Oncol 21:387-390, 2000.
13. Anderson J, Behbakht K, DeGeest K, et al: Adenosarcoma in a patient with vaginal endometriosis. Obstet Gynecol 98:964-966, 2001.
14. Kerner H, Lichtig C: Mullerian adenosarcoma presenting as cervical polyps: A report of seven cases and review of the literature. Obstet Gynecol 81:655-659, 1993.
15. Clement PB, Oliva E, Young RH: Mullerian adenosarcoma of the uterine corpus associated with tamoxifen therapy: A report of six cases and a review of tamoxifen associated endometrial lesions. Int J Gynecol Pathol 15:222-229, 1996.
16. Eichhorn JH, Young RH, Clement PB, et al: Mesodermal (mullerian) adenosarcoma of the ovary: A clinicopathologic analysis of 40 cases and a review of the literature. Am J Surg Pathol 26:1243-1258, 2002.
17. Amant F, Gabriele C, Moerman P, et al: Letters to the Editor. Gynecol Oncol 88:463-464, 2003.