Neoadjuvant Therapy for Gastric Cancer
Gastric cancer is a global health issue. Most cases are diagnosed atan advanced stage with poor prognosis. Current therapies have a modestimpact on survival. Surgery remains the only potentially curativetreatment, but is associated with a high rate of locoregional recurrenceand distant metastases. Total gastrectomy for proximal cancers is complicatedby postoperative morbidity and quality-of-life impairment.Combined-modality therapy may improve outcomes in this disease.Adjuvant therapy for gastric cancer has now become the standard inthe Western world. However, adjuvant therapy improves survival by onlya few months and is associated with high morbidity. Neoadjuvant therapyis commonly used for esophageal and gastroesophageal junction cancers,but is still regarded as investigational in gastric cancer. Severalsmall phase II studies indicate the feasibility of neoadjuvant strategies.The incorporation of novel, targeted agents into neoadjuvant programsand an assessment of biologic changes within the tumor may refinetherapy. This article provides a concise review of the literature onneoadjuvant therapy for gastric cancer and suggests avenues for furtherinvestigation.
Gastric cancer is a global health issue. Most cases are diagnosed at an advanced stage with poor prognosis. Current therapies have a modest impact on survival. Surgery remains the only potentially curative treatment, but is associated with a high rate of locoregional recurrence and distant metastases. Total gastrectomy for proximal cancers is complicated by postoperative morbidity and quality-of-life impairment. Combined-modality therapy may improve outcomes in this disease. Adjuvant therapy for gastric cancer has now become the standard in the Western world. However, adjuvant therapy improves survival by only a few months and is associated with high morbidity. Neoadjuvant therapy is commonly used for esophageal and gastroesophageal junction cancers, but is still regarded as investigational in gastric cancer. Several small phase II studies indicate the feasibility of neoadjuvant strategies. The incorporation of novel, targeted agents into neoadjuvant programs and an assessment of biologic changes within the tumor may refine therapy. This article provides a concise review of the literature on neoadjuvant therapy for gastric cancer and suggests avenues for further investigation.
Gastric cancer is a global health issue. Worldwide, it is the second most common cause of cancer-related mortality.[1] In the United States, gastric cancer is the eighth most common cause of cancer- related death.[2] Cancer of the gastric antrum has decreased in incidence in the United States and western Europe; however, this has been countered by an increased incidence of cancer of the gastric cardia.[3-5] In the Far East, particularly Japan, there has been a notable improvement in mortality associated with this disease.[ 6] This has been partially attributed to early detection of tumors by screening and surveillance programs. Cancers of the distal stomach are more common in Asia, and exhibit the following characteristics: (1) They are associated with Helicobacter pylori infection, (2) they follow a chronic atrophic gastritis/metaplasia/dysplasia sequence, and (3) upon histology, they are usually the intestinal type.[1,7] On the other hand, cancers of the proximal stomach have been associated with improved socioeconomic conditions and are less often associated with H pylori.[1,7] Proximal tumors are associated with early hematogenous spread as compared to slower progression and locoregional spread in the case of distal tumors.[7] There has been a recent increase in the incidence of proximal gastric and gastroesophageal junction cancers. The reasons for this increase are unknown.[ 3-5] Siewert and Stein classified adenocarcinoma of esophagogastric junction (AEG) into three distinct entities based on anatomic origin: (1) adenocarcinoma of the distal esophagus invading the gastroesophageal junction (AEG type I tumors), (2) carcinoma of the gastric cardia immediately at the gastroesophageal junction (AEG type II tumors), and (3) subcardial gastric carcinoma infiltrating the gastroesophageal junction (AEG type III tumors).[8] AEG type II and III tumors are more likely to be undifferentiated and have a worse prognosis.[1] Thus, although gastric cancer is referred to as one entity, there is heterogeneity due to epidemiologic, clinical, and therapeutic differences between cardiac, antral, and gastroesophageal junction cancers.
In the United States and Europe, potentially curative resections (with negative margins, R0) are possible in only about 50% of newly diagnosed gastric cancer patients.[9-11] For patients with locally advanced tumors (stages II, IIIA, or IIIB) even after gastric resection with curative intent, the recurrence rate is as high as 40% to 65%.[12] As most cases are diagnosed at an advanced stage, there is clearly a need to develop innovative treatment strategies that will downstage the tumor, increase the R0 resection rate, and decrease the risk of recurrence after surgery. Adjuvant chemoradiation is commonly used postoperatively for gastric cancer. The ability to deliver adjuvant chemoradiation is adversely influenced by the 25% to 46% postoperative morbidity seen after gastrectomy with lymphadenectomy.[13,14] Only 64% of patients completed adjuvant chemoradiotherapy in the important Intergroup 0116 trial.[15] Neoadjuvant therapy for gastric cancer is emerging as a promising approach. This article discusses the rationale for neoadjuvant therapy and the available therapeutic options. A brief overview of current staging and operative and adjuvant strategies is presented below as a preface to this discussion. Staging Strategies
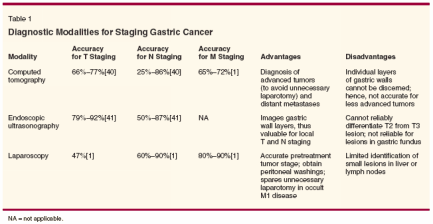
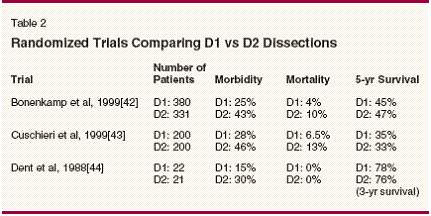
National Comprehensive Cancer Network (NCCN) guidelines recommend a multidisciplinary approach to staging that includes a complete history and physical examination, laboratory studies (complete blood count, platelet count, and serum biochemistry), esophagogastroduodenoscopy, chest radiography, and computed tomography (CT) of the abdomen and pelvis. Laparoscopy has also been added to these guidelines as a category 2B recommendation (nonuniform consensus among panel members).[16] Table 1 depicts the various staging modalities available for gastric cancer, along with accuracy, advantages, and disadvantages. The use of positronemission tomography (PET) for staging, detecting recurrence, and evaluating response to therapy in gastric cancer is evolving. A recent study in esophageal cancer suggests that quantitative measurements of tumor 18F-fluorodeoxyglucose (FDG) uptake may predict histopathologic tumor response and patient outcome as early as 2 weeks after initiation of preoperative chemotherapy.[ 17] This may be a useful investigational tool in gastric cancer as part of a neoadjuvant strategy. The recent availability of integrated CT/PET imaging is likely to refine staging of these tumors in the future. Japanese investigators use a computer- based program (the Maruyama program) to predict lymph node stations at risk for metastasis in particular cases. The cases are sorted on the basis of similarity with seven specified variables: age (± 5 years), sex, Borrmann type of the tumor, greatest dimension of the tumor as measured on the luminal surface (± 2.5 cm), location of the tumor, estimated tumor depth, and histology. Comparison is made with a database of 3,843 gastric cancer patients at the National Cancer Center Hospital in Tokyo who had been treated by D2 or more extensive lymphadenectomy. The program then predicts the percentage likelihood of disease at each of the 16 lymph node stations around the stomach on the basis of actual patient experience.[18] This information could be used prospectively to enroll higher-stage patients into neoadjuvant programs. The Role of Surgery Surgical resection remains the only curative therapy for this disease. The debate continues regarding the optimal extent of lymph node dissection required for maximal therapeutic benefit. Extended lymphadenectomy remains the standard of care in the Far East at the cost of acceptable postoperative morbidity.[19] Similar results could not be reproduced in Western trials and no survival advantage was attributed to D2 dissections in the Dutch Gastric Cancer Group trial.[14] Similar results were noted in the Medical Research Council (MRC) trial from the United Kingdom.[13] The results of randomized control trials comparing D1 with D2 dissections are depicted in Table 2. These randomized trials did not reveal any survival advantage for D2 dissection. Morbidity and mortality associated with D2 dissection was significantly higher in these randomized trials. Five-year survival after D1/D2 dissection remains dismal at 30% to 45%. Thus, no phase III study in the Western population has proved the therapeutic advantage of D2 dissection. Cochrane database review done by McCulloch et al revealed survival benefit of D2 dissections in T3 or more advanced lesions.[20] Subgroup analysis of the MRC trial suggests a survival advantage for stage II and III patients or those with N1 disease.[13] The Dutch Gastric Group trial indicated a survival benefit for N2 patients.[ 14] Siewert et al, in a large prospective multicenter observational trial, found that the pathologic subgroup of pT2, N1 and pT3, N0 had a significant survival benefit with extended lymph node dissection.[21] The extent of gastrectomy may also impact on postoperative morbidity and quality of life. Due to high incidence of locoregional failure, multifocal disease, and submucosal pattern of spread, total gastrectomy with at least a 5-cm gastric margin is recommended for proximal gastric tumors.[9] Subtotal gastrectomy is often the procedure of choice for distal (antral) tumors.[22] Although total gastrectomy is superior in terms of margin-free resection, this procedure is associated with postoperative morbidity of dumping syndrome, malabsorption, anemia, osteoporosis, and malnutrition. Quality- of-life impairment results from these difficulties.
Davies et al demonstrated that gastric cancer patients who underwent subtotal gastrectomy enjoyed superior quality of life at the end of 1 year as compared with total gastrectomy patients.[23] Proximal gastric resection is associated with a higher risk of anastomotic leakage and bile-acid reflux. This complication may be prevented by jejunal interposition.[9] Based on the above observations, it may be worthwhile to investigate neoadjuvant therapy followed by proximal gastrectomy as a possible alternative to total gastrectomy for proximal gastric cancers. A recent trial by Bosing et al revealed a dismal 14% 5-year survival in patients with AEG type III tumors, despite surgery.[24] Linitis plastica and signet-ring histology are other gastric cancer subtypes that have a poor outcome with surgical therapy alone.[25] Thus, patients with stages II, IIIA, or IIIB (locally advanced) proximal tumors or those with aggressive histology are likely to benefit from neoadjuvant therapy, as surgery alone is usually not curative in these cases. The Role of Adjuvant Therapy The use of postoperative adjuvant chemoradiotherapy for gastric cancer gained popularity after a randomized trial by Macdonald et al.[15] The trial showed improved survival in the adadjuvant therapy arm receiving 45 Gy of radiotherapy and three cycles of fluorouracil (5-FU) and leucovorin compared to surgery alone. Median overall survival in the surgery-only group was 27 months, as compared with 36 months in the chemoradiotherapy group (P = .005). Critics of this trial note that over half of the patients received inadequate lymphadenectomy (most had D0 dissection); hence, adjuvant therapy may have been advantageous for these patients, many of whom possibly had residual nodal disease. In the Western world, adjuvant therapy is considered standard of care since the rate of R0 resection (microscopically negative postoperative margins) remains suboptimal. The role of adjuvant therapy after D2 dissection is unproven. Neoadjuvant Therapy
Neoadjuvant therapy has a number of theoretical advantages. First, neoadjuvant therapy may shrink and "downstage" the tumor, hence allowing complete resection of cancers previously considered inoperable. Second, radiation treatment volumes are more precise and tissues are better oxygenated when radiation is administered preoperatively. Third, postoperative morbidity and significant weight loss of up to 10% precludes administration of adjuvant therapy in more than 30% of patients.[26] Neoadjuvant therapy, including chemotherapy and radiation, is better tolerated and feasible in most patients. This strategy offers patients the maximum benefit of all available modalities of treatment.
Fourth, neoadjuvant therapy provides a unique mechanism to assess in vivo response to chemotherapeutic agents and an opportunity to study molecular changes with therapy. Neoadjuvant therapies may include preoperative chemotherapy, preoperative external-beam radiation therapy (EBRT), concurrent chemoradiotherapy (chemo-RT), and intraperitoneal chemotherapy. These modalities are discussed below. Preoperative Chemotherapy
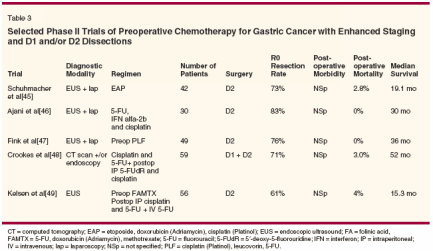
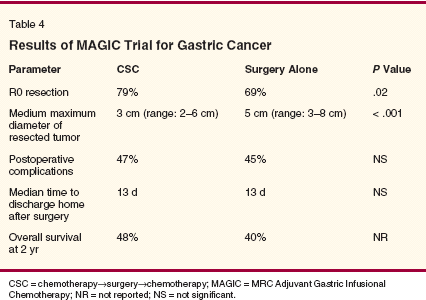
Selected phase II trials using preoperative chemotherapy are shown in Table 3. Studies that reported median overall survival and included either D2 dissection or comprehensive staging workup were selected. These trials demonstrated that chemotherapy delivered in the preoperative setting is feasible and well-tolerated. These small trials suggest an improved R0 resection rate and a favorable median survival compared with historical controls (median survival of patients who underwent surgery alone in the Intergroup 0116 trial was 27 months).[15] An observation by Lowy et al was that the chemotherapy responders had a twofold improvement in overall survival compared to nonresponders.[27] Although promising, a final answer regarding the efficacy of neoadjuvant chemotherapy can only be provided by a well-powered phase III study. Currently, three large phase III trials investigating the role of neoadjuvant therapy are under way. These include the MRC Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial, the Swiss Study SAKK 43/99, and the multinational European trial EORTC (European Organisation for Research and Treatment of Cancer) 40954.[13,28] The MAGIC trial accrued 503 gastric cancer patients between 1994 and 2002.[13] Patients had stage II or more advanced gastric cancer and were randomized to perioperative chemotherapy with three preoperative and three postoperative cycles of epirubicin (Ellence), cisplatin, and 5-FU (the CSC [chemotherapy->surgery->chemotherapy] arm) or surgery alone. The preliminary results of the trial are depicted in Table 4. The R0 resection rate was significantly higher in the CSC arm with no significant difference in postoperative complications or length of postoperative hospital stay. Progression-free survival was superior in the CSC arm as compared with the surgery-alone arm (hazard ratio = 0.70, 95% confidence interval [CI] = 0.56-0.88, P = .002). The trend toward improved survival awaits trial completion.
Two elements of the study design of the MAGIC trial deserve mention. First, about 11% of patients had distal esophageal cancer. A prior Intergroup study in esophageal cancer revealed no survival advantage with preoperative chemotherapy; however, there are limited data in gastric cancer.[29] This may confound study results. In addition, neither endoscopic ultrasonography nor diagnostic laparoscopy was performed to accurately assess stage of disease preoperatively. Despite these problems, the MAGIC trial is the first well-powered, randomized, controlled phase III trial that demonstrates the safety and feasibility of perioperative chemotherapy. Final efficacy results of this study are eagerly anticipated. The Swiss study SAKK 43/99 compares preoperative docetaxel (Taxotere), cisplatin, and 5-FU followed by surgery, vs surgery followed by the same chemotherapy regimen. The EORTC 40954 trial compares surgery plus preoperative cisplatin, leucovorin, and 5-FU with surgery alone. The results of these trials will help establish whether there is a benefit of neoadjuvant chemotherapy and may provide information regarding the superiority of specific neoadjuvant chemotherapy regimen. Preoperative EBRT
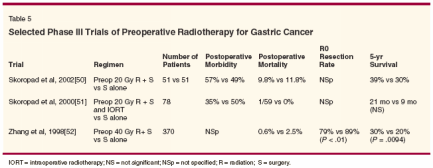
The rationale for neoadjuvant radiation is reduction of local and regional recurrences. External-beam radiation therapy also has a palliative role for the management of bleeding tumors. The location and extent of tumor is best imaged in the preoperative setting. As mentioned earlier, delivery of radiation therapy preoperatively may enhance efficacy, reduce treatment volumes, and decrease toxicity. Almost one-third of the radiation fields had to be redesigned in the postoperative adjuvant setting in the Intergroup 0116 trial.[15] Preoperative EBRT allows the patient a better chance of receiving all available therapies, as postoperative morbidity after gastrectomy is not insignificant. Table 5 shows trials comparing preoperative radiation plus surgery with surgery alone. These studies used EBRT doses between 20 and 40 Gy. However, more current combinedmodality regimens utilize EBRT doses up to 45 Gy (Table 6). Zhang et al demonstrated a significant improvement in 5-year survival after 40 Gy was given preoperatively.[52] There did not appear to be an increase in postoperative morbidity related to the therapy; these studies indicated a trend toward improved survival. Preoperative Chemo-RT
Given the benefit of single-modality treatment (chemotherapy or radiation) preoperatively in gastric cancer and the experience using preoperative chemo-RT in other gastrointestinal malignancies, such as esophageal and rectal cancer, preoperative chemo- RT strategies for gastric cancer appear logical. However, experience in this area is rather limited. Trials investigating preoperative chemo-RT for gastric cancer are depicted in Table 6. These studies indicate that combined-modality therapy, consisting of chemo-RT followed by surgery, is well tolerated, without significant increase in morbidity or mortality. These studies utilized cisplatin and 5-FU- based regimens. It is possible that use of newer radiation sensitizers such as docetaxel, irinotecan (Camptosar), oxaliplatin (Eloxatin), or gemcitabine (Gemzar), alone or in combination, may yield higher pathologic responses.
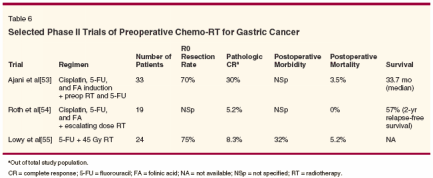
In these studies, complete response was correlated with improved survival. The trial by Ajani et al (Table 3) deserves special mention.[46] These investigators utilized a three-step approach in which patients first received two cycles of chemotherapy consisting of 5-FU and cisplatin, followed a month later by EBRT of 45 Gy and infusional 5-FU prior to surgery. The approach yielded an encouraging 70% R0 resection rate and a pathologic complete response rate of 30%.The median survival in this study population was 33.7 months, with a median survival time of 63.9 months in patients with a complete response. The trial results support the hypothesis that a two-step preoperative approach leads to superior pathologic response, which in turn leads to improved clinical outcome. Will aggressive neoadjuvant approaches allow limited gastric resection? Future phase III trials are necessary to answer this question. Perioperative Intraperitoneal Therapy
Intraperitoneal chemotherapy is an attractive treatment modality for gastric cancer for several reasons. Even after extended lymphadenectomy, the peritoneum remains a major site of failure. Locoregional recurrence rates are as high as 40% to 50%, suggesting that peritoneal implantation is frequent in this cancer.[30] It is also postulated that manipulation of gastric cancer during surgery may result in the release of free cells into the peritoneal cavity; these cells get trapped in fibrin and proliferate under the influence of cytokines released during surgical stress and wound healing. Using intraperitoneal chemotherapy in the perioperative period can eradicate tumor cells prior to entrapment in the fibrin and development of fibrosis. Because hyperthermia and chemotherapy have been shown to be synergistic, perioperative intraperitoneal hyperthermic chemoperfusion (IHCP) may be advantageous for management of gastric cancer.[31,32] Continuous IHCP can be performed following temporary closure of the abdomen or with an open abdomen technique. Peritoneal perfusate is maintained at 41oC to 42oC and uniform drug distribution is ensured mechanically.[30] Selected randomized control trials of perioperative intraperitoneal chemotherapy in locally advanced gastric cancers with R0 resection are shown in Table 7. Sugarbaker et al performed a meta-analysis of perioperative intraperitoneal therapy trials.[ 30] Their data suggest improved overall survival in the perioperative intraperitoneal chemotherapy group vs surgery alone. Maximal benefit was noted in stage III or IV gastric cancer. As seen earlier, some trials of preoperative chemotherapy (Table 3) also included postoperative intraperitoneal therapy. This is an interesting approach that can be considered for gastric cancer patients with a high likelihood of peritoneal dissemination, such as those with linitis plastica or locally advanced signet-ring carcinoma of the stomach. Clearly, administration of intraperitoneal therapy is complicated and adds some morbidity. Therefore, this approach should be limited to centers with high levels of expertise in the context of a clinical trial. New Approaches It is clear that the efficacy of traditional chemotherapeutic agents is limited in gastric cancer. Newer agents such as docetaxel, irinotecan, oxaliplatin, and capecitabine (Xeloda) are active in metastatic gastric cancer and may represent promising options for neoadjuvant therapy.[33-38] Another agent of interest is pemetrexed (Alimta), which is a multitargeted antifolate that inhibits multiple enzymes important in folate metabolism. Preliminary results of a phase II investigation of single-agent pemetrexed in patients with locally advanced or metastatic gastric cancer showed a response rate of 28% with an acceptable adverse event profile in patients treated with folic acid and B12 supplementation.[39] Novel agents including trastuzumab (Herceptin), cyclooxygenase inhibitors, antiangiogenic agents, matrix metalloproteinase inhibitors, and EGFR-specific inhibitors such as gefitinib (Iressa), erlotinib (Tarceva), and cetuximab (Erbitux) are currently being investigated in gastric cancer. These agents offer hope for the development of more effective regimens, both as single agents and in combination. Once their activity in gastric cancer is established, studies incorporating these agents into neoadjuvant strategies should be conducted. In the future, neoadjuvant approaches may need to be tailored to the individual patient. The use of metabolic- based imaging such as FDGPET may identify early responders and thereby serve to exclude patients from further ineffective systemic therapy. Such patients can then go on to receive immediate surgery. Further, patients who progress on neoadjuvant therapy may be spared aggressive surgical intervention, as this subgroup historically does very poorly regardless of therapy. The availability of tissue pre- and postchemotherapy with neoadjuvant therapy allows the possipossibility of performing proteomic and other biologic assays which may help further to refine this therapy. Conclusions Current data suggest the possibility of diverse neoadjuvant strategies that are worthy of further investigation for the management of gastric cancer. These strategies include neoadjuvant chemotherapy, chemoradiation, radiotherapy, and perioperative intraperitoneal therapy. At this point in time, it is unclear which of these options offer therapeutic superiority. Choice of an individual modality is dictated by clinical presentation. Neoadjuvant chemoradiation improves R0 resection rates and should be considered for large, proximal tumors that cannot be removed with a margin- free resection. This therapy, in combination with limited surgical resection, may be considered for proximal gastric cancer patients who are not candidates for total gastrectomy. Perioperative intraperitoneal therapy can be considered for positive peritoneal cytology. External-beam radiation therapy is useful for palliation of bleeding gastric tumors. Incorporation of targeted approaches may further improve the outcome of gastric cancer patients. Results of ongoing phase III trials in this regard are eagerly awaited.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Hohenberger P, Gretschel S: Gastric cancer. Lancet 362:305-315, 2003.
2. Jemal A, Tiwari RC, Murray T, et al: Cancer statistics, 2004. CA Cancer J Clin 54:8-29, 2004.
3. Powell J, McConkey CC: Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer 62(3):440- 443, 1990.
4. Zheng T, Mayne ST, Holford TR, et al: The time trend and age-period-cohort effects on incidence of adenocarcinoma of the stomach in Connecticut from 1955-1989. Cancer 72(2):330-340, 1993.
5. Blot WJ, Devesa SS, Kneller RW, et al: Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 265:1287- 1289, 1991.
6. Lambert R, Guilloux A, Oshima A, et al: Incidence and mortality from stomach cancer in Japan, Slovenia and the USA. Int J Cancer 97(6):811-818, 2002.
7. Alberts SR, Cervantes A, van de Velde CJH: Gastric cancer: Epidemiology, pathology and treatment. Ann Oncol 14(suppl 2):ii31-ii36, 2003.
8. Siewert JR, Stein HJ: Adenocarcinoma of the gastroesophageal junction: Classification, pathology and extent of resection. Dis Esoph 9:173-182, 1996.
9. Bonenkamp J: Surgery for upper gastrointestinal malignancies. Semin Oncol 31(4):542-553, 2004.
10. Wanedo HJ, Kennedy BJ, Chmiel J, et al: Cancer of the stomach: A patient care study by the American College of Surgeons. Ann Surg 218:583-592, 1993.
11. Adashek K, Sanger J, Longmire WP: Cancer of the stomach: Review of the consecutive ten-year intervals. Ann Surg 198:6-10, 1979.
12. Landry J, Tepper JE, Wood WC, et al: Patterns of failure following curative resection of gastric cancer. Int J Radiat Oncol Biol Phys 19:1357-1362, 1990.
13. Cunningham D, Allum W, Weeden S: Perioperative chemotherapy in operable gastric and lower oesophageal cancer: A randomised, controlled trial of the UK NCRI Upper GI Clinical Studies Group (the MAGIC trial, ISRCTN 93793971). Eur J Cancer 1(5 suppl):S18, 2003.
14.Hartgrink HH, van de Velde CJ, Putter H, et al: Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 22(11):2069-2077, 2004.
15. Macdonald JS, Smalley S, Benedetti J, et al: Chemotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725-730, 2001.
16. Available at National Comprehensive Cancer Network website (http://www.nccn.org). Accessed July 12, 2005.
17. Weber WA, Ott K: Imaging of esophageal and gastric cancer. Semin Oncol 31(4):530-541, 2004.
18. Guadagni S, de Manzoni G, Catarci M, et al: Evaluation of the Maruyama computer program accuracy for preoperative estimation of lymph node metastases from gastric cancer. World J Surg 24(12):1550-1558, 2000.
19. Sano T, Katai H, Sasako M, et al: One thousand consecutive gastrectomies without operative mortality. Br J Surg 89:123, 2002.
20. McCulloch P, Nita ME, Kazi H, et al: Extended versus limited lymph node dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev (4)CD001964, 2003.
21. Siewert JR, Bottcher K, Stein H, et al: Relevant prognostic factors in gastric cancer: Ten-year results of the German Gastric Cancer Study. Ann Surg 228(4):449-461, 1998.
22. Bozzetti F, Marubini E, Bonfanti G, et al: Subtotal versus total gastrectomy for gastric cancer: Five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg 230(2):170-178, 1999.
23. Davies J, Johnston D, Sue Ling H, et al: Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg 22:1048-1055, 1998.
24. Bosing NM, Heise JW, Goretzki PE, et al: Adenocarcinoma of the esophagogastric junction: Prognostic factors and results of primary surgery [in German]. Chirurg 75(11):1088-1097, 2004.
25. Hamy A, Letessier E, Bizouarn P, et al: Study of survival and prognostic factors in patients undergoing resection for gastric linitis plastica: A review of 86 cases. Int Surg 84(4):337-343, 1999.
26. Yu W, Seo BY, Chung HY: Postoperative body-weight loss and survival after curative resection for gastric cancer. Br J Surg 89:467-470, 2002.
27. Lowy AM, Mansfield PF, Leach SD, et al: Response to neoadjuvant chemotherapy best predicts survival after curative resection for gastric cancer. Ann Surg 229:303-308, 1999.
28. Fink U: EORTC Protocol 40 954. Randomized phase III study of preoperative chemotherapy followed by surgery versus surgery alone in locally advanced gastric cancer. Brussels, European Organisation for Research and Treatment of Cancer, 1999.
29. Kelsen DP, Ginsberg R, Pajak TF, et al: Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 339(27):1979-1984, 1998.
30. Sugarbaker PH, Yu W, Yonemura Y: Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: The evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol 21:233-248, 2003.
31. Barlogie B, Corry PM, Drewinko B: In vitro thermochemotherapy of human colon cancer cells with cis-dichlorodiamineplatinum (II) and mitomycin C. Cancer Res 40:1165-1168, 1980.
32. Teicher BA, Kowal CD, Kennedy KA, et al: Enhancement by hyperthermia of the in vitro cytotoxicity of mitomycin C toward hypoxic tumor cells. Cancer Res 41:1096-1099, 1981.
33. Bleiberg H: CPT-11 in gastrointestinal cancer. Eur J Cancer 35:371-379, 1999.
34. Narahara H, Uedo U, Fujitani K, et al: Phase II study of CPT-11 plus docetaxel in patients with metastatic gastric cancer: Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG 0101). Proc Am Soc Clin Oncol 22:374a, 2003.
35. Do-Youn O, Kwon JH, Lee JJ, et al: Docetaxel + 5-fluorouracil + cisplatin combination chemotherapy as a first-line treatment in inoperable or relapsed gastric cancer. Proc Am Soc Clin Oncol 22:342a, 2003.
36. Mavroudis D, Kourousis C, Androulakis N, et al: Frontline treatment of advanced gastric cancer with docetaxel and granulocyte colony-stimulating factor (G-CSF): A phase II trial. Am J Clin Oncol 23:341-344, 2000.
37. Sulkes A, Smyth J, Sessa C, et al: Docetaxel (Taxotere) in advanced gastric cancer: Results of a phase II clinical trial. EORTC Early Clinical Trials Group. Br J Cancer 70:380-383, 1994.
38. Louvet C, Andre T, Tigaud JM, et al: Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol 20:4543- 4548, 2002.
39. Celio L, Bajetta E, Buzzoni R, et al: Efficacy and toxicity of pemetrexed disodium (Alimta) with folic acid (FA) in gastric cancer. Proc Am Soc Clin Oncol 20:166a, 2001.
40. Abdalla EK, Pisters PWT: Staging and preoperative evaluation of upper gastrointestinal malignancies. Semin Oncol 31:513-529, 2004.
41. Rösch T: Endosonographic staging of gastric cancer: A review of literature results. Gastrointest Endosc Clin N Am 5:549-557, 1995.
42. Bonenkamp JJ, Sasako M, Hermans J, et al: Extended lymph node dissection for gastric cancer. N Engl J Med 340:908-914, 1999.
43. Cuschieri A, Weeden S, Fielding J, et al: Patient survival after D1 and D2 resections for gastric cancer: Long-term results of the MRC randomized surgical trial. Br J Cancer 79:1522- 1530, 1999.
44. Dent DM, Madden MV, Price SK: Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg 75:110-112, 1988.
45. Schuhmacher CP, Fink U, Becker K, et al: Neoadjuvant therapy for patients with locally advanced gastric carcinoma with etoposide, doxorubicin, and cisplatinum. Closing results after 5 years of follow up. Cancer 91:918-927, 2001.
46. Ajani JA, Mansfield PF, Lynch PM, et al: Enhanced staging and all chemotherapy preoperatively with potentially resectable gastric carcinoma. J Clin Oncol 17:2403-2411, 1999.
47. Fink U, Ott K, Dittler HJ, et al: Neoadjuvant cisplatinum, leucovorin and fluorouracil (PLF) in adequately staged patients with locally advanced gastric carcinoma. Proc Am Soc Clin Oncol 18:272, 1999.
48. Crookes P, Leichman CG, Leichman L, et al: Systemic chemotherapy for gastric carcinoma followed by postoperative intraperitoneal therapy: A final report. Cancer 79:1767-1775, 1997.
49. Kelsen D, Karpeh M, Schwartz G, et al: Neoadjuvant therapy of high risk gastric cancer: A phase II trial of preoperative FAMTX and postoperative intraperitoneal fluorouracilcisplatinum plus intravenous fluorouracil. J Clin Oncol 14:1818-1828, 1996.
50. Skoropad V, Berdov B, Zagrebin V: Concentrated preoperative radiotherapy for resectable gastric cancer: 20-year follow-up of a randomized trial. J Surg Oncol 80(2):72-78, 2002.
51. Skoropad VY, Berdov BA, Mrdynski YS, et al: A prospective, randomized trial of preoperative and intraoperative radiotherapy versus surgery alone in resectable gastric cancer. Eur J Surg Oncol 26:773-779, 2000.
52. Zhang ZX, GU XZ, Yin WB, et al: Randomized clinical trial on the preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC) Report on 370 patients. Int J Radiat Oncol Biol Phys 42:929-934, 1998.
53. Ajani JA, Mansfield PF, Janjan N, et al: Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol 22(14):2774-2780, 2004.
54. Roth AD, Allal AS, Brundler MA, et al: Neoadjuvant radiochemotherapy for locally advanced gastric cancer: A phase I-II study. Ann Oncol 14(1):110-115, 2003.
55. Lowy AM, Feig BW, Janjan N, et al: A pilot study of preoperative chemoradiotherapy for resectable gastric cancer. Ann Surg Oncol 8:519-524, 2001.
56. Kim JY, Bae HS: A controlled clinical study of serosa-invasive gastric carcinoma patients who underwent surgery plus intraperitoneal hyperthermo-chemo-perfusion (IHCP). Gastric Cancer 4(1):27-33, 2001.
57. Yu W, Whang I, Chung HY, et al: Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: Results of a prospective randomized trial. World J Surg 25(8):985-990, 2001.
58. Fujimoto S, Takahashi M, Mutou T, et al: Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postop- erative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 85(3):529- 534, 1999.
59. Yonemura Y, Ninomiya I, Kaji M, et al: Prophylaxis with intraoperative chemohyperthermia against peritoneal recurrence of serosal invasion-positive gastric cancer. World J Surg 19(3):450-454, 1995.
60. Hamazoe R, Maeta M, Kaibara N: Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 73(8):2048-2052, 1994.