Mohs Micrographic Surgery: Established Uses and Emerging Trends
Mohs surgery has been well-established as the gold standard for the treatment of BCCs and SCCs. And, as described in this article, preliminary reports suggest that it may play an equally important role in the management of several other cutaneous malignancies.
Mohs micrographic surgery is a surgical technique that seeks to ensure the clearance of cutaneous tumors while maximizing normal tissue conservation. This is accomplished through the sequential removal of thin layers of tissue in which the entire peripheral and deep margins are examined for residual tumor. This approach appears to be superior to conventional surgical excision in the treatment of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), the two most common cancers of the skin. Its efficacy in treating BCC and SCC has led clinicians to explore the role of Mohs micrographic surgery in the management of less common cutaneous neoplasms, such as melanoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, extramammary Paget’s disease, and microcystic adnexal carcinoma.
Mohs micrographic surgery is a specialized surgical technique in which meticulous microscopic mapping of residual tumor guides the surgeon in complete extirpation of the cutaneous malignancy. In doing this, the technique seeks to ensure complete tumor eradication while maximizing normal tissue conservation. This approach appears to be superior to conventional surgical excision in the management of the most common cutaneous neoplasms, namely basal cell carcinoma (BCC)[1,2] and squamous cell carcinoma (SCC).[3]
The increased cure rates for nonmelanoma skin cancer observed with Mohs surgery are largely attributable to the complete microscopic margin examination that occurs with its horizontal sectioning of the specimens. This is not the case with standard surgical excisions, as the majority of surgical pathologists continue to employ either cross-sectioning or bread-loaf sectioning to process tissue specimens. With these methods, less than 1% of the excised margin of the tumor is actually visualized.[4] As a result, the potential for unrecognized margin involvement is much higher with step sectioning of tissue than with the Mohs technique. This difference translates into higher rates of tumor recurrence for standard excision
History
The field of Mohs micrographic surgery has evolved considerably since its conceptualization in 1936 by Dr. Frederic Mohs. The idea originated from Dr. Mohs' and others' work with the in vivo application of chemical cauterants to tumors of the skin for nonselective destruction. His observation of the unique in vivo fixative properties of one particular escharotic agent, zinc chloride paste, led him to the idea of microscopically controlled excision of tumors.[5]
In Mohs' original technique, which was known as chemosurgery, zinc chloride paste was applied to the skin cancer and allowed to sit overnight. The patient returned the following day for surgical extirpation of the tumor followed by microscopic examination of horizontal sections of the specimen for margin clearance. The major limitations of the procedure were significant pain associated with application of the zinc chloride, prominent tissue inflammation secondary to the cauterant precluding reconstruction of defect, and the time-intensive nature of the 2-day procedure. The development of the fresh-tissue technique in 1974 by Stegman and Tromovitch allowed the procedure to be completed in 1 day and eliminated the pain and inflammation associated with use of zinc chloride.[6] Since this innovation, Mohs surgery has become the gold standard for managing nonmelanoma skin cancers.
Basic Technique
Mohs micrographic surgery begins with clinical identification by the physician of tumor margins through visual examination, palpation, and gentle curettage. After the surgical site is anesthetized with a local anesthetic, an incision is made with a scalpel just outside the clinically identifiable margins of the tumor. The distance between the clinical border of the neoplasm and the incision is variable, and depends on the type of malignancy, its size, and how well demarcated it is. In contrast to a traditional cutaneous excision, the incision is typically made at an angle of 30° to 45° with the skin such that the resulting excised specimen has a beveled edge to facilitate processing. At the time of incision, score marks are placed in both the specimen and the defect site for tissue orientation.
The tissue specimen is then transported to the histopathology laboratory, where the specimen is divided into smaller pieces based on the score marks and inked to preserve tissue orientation. A map correlating the surgical defect to the inked specimens is generated. In our laboratory, tissue specimens are pressed onto a glass slide and then freeze-mounted on a tissue chuck. This tissue flattening permits the tangential sectioning of the tissue, which makes Mohs surgery possible. The chucks are then placed in the cryostat for sectioning of the tissue. This yields 6- to 8-μm sections of the entire peripheral margins of the specimen including the base of the lesion. The slides are then stained with hematoxylin and eosin or, more rarely, toluidine blue. The generation of interpretable, highquality frozen section slides is a technically demanding task, which is highly dependent on the skill and experience of the histotechnician.
Next, the Mohs surgeon examines the slides for any evidence of tumor. If residual tumor is identified on the slides, the location is marked on the tissue map. This map is then returned to the patient's bedside for identification of the corresponding region of tumor involvement in the defect site. This area is subsequently removed for further tissue processing and examination, as described above. The process is repeated until the peripheral and deep margins are histologically free of tumor.
The final step in this process involves evaluation of the wound for second-intention healing vs reconstruction of the defect. Many factors enter into the appropriate determination at this juncture. These include the biologic nature of the malignancy removed, preservation of form and function of surrounding tissues, optimization of cosmesis, and patient comorbidities.
Use in Specific Tumor Types
Basal Cell and Squamous Cell Carcinomas
FIGURE 1
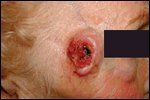
Basal Cell Carcinoma
BCC and SCC are the two most commonly occurring cancers in the United States. Together, they comprise over 95% of all nonmelanoma skin cancers, accounting for over 1 million reported cases in 2004.[7] Both tumors occur with increased frequency in fairskinned populations; however, BCCs outnumber SCCs by approximately 4:1. Exposure to ultraviolet radiation has repeatedly been identified as the single most significant factor in the etiology of both neoplasms. As a result, sunexposed areas of the skin are the most common locations of each tumor, with over 80% occurring on the face, scalp, neck, or dorsal hands. BCCs typically present as pearly pink papules with a rolled border and variable central ulceration (Figure 1). SCCs usually arise as a firm, erythematous hyperkeratotic papule or plaque (Figure 2).
Mohs micrographic surgery remains the gold standard for the surgical management of basal cell and squamous cell carcinomas. A review of over 10,000 cases of primary BCC treated with either standard surgical excision and traditional margin assessment or Mohs surgery revealed a long-term (> 5 year) recurrence rate of 10.1% for surgical excision vs 1.0% for Mohs surgery.[1] For the management of recurrent BCC, the long-term recurrence rate for Mohs surgery was 5.6%, as compared to 9.8% for radiation therapy and 17.4% for surgical excision.[2]
FIGURE 2
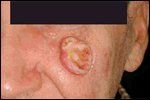
Squamous Cell Carcinoma
Equally superior results have been documented for the treatment of SCC with Mohs micrographic surgery (Figures 3 and 4). In a review of cases of SCC in the literature, Rowe et al reported recurrence rates of 3.1% for Mohs surgery vs 10.9% for surgical excision. For recurrent tumors, Mohs surgery yielded a recurrence rate of 10.0%, whereas surgical excision led to recurrences in 23.3% of cases.[3]
Melanoma and Melanoma In Situ
FIGURE 3
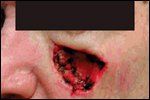
Immediate Postsurgical Result
Cutaneous melanoma is a neoplasm derived from melanocytes of the epidermis with the potential for both aggressive local growth and systemic spread. Melanoma in situ refers to a subtype of melanoma in which the neoplastic melanocytes are confined to the epidermis and associated adnexal structures without evidence of disease invading the adjacent dermis. The tumor typically presents as a brown to black patch, papule, or plaque with significant color variegation and border irregularity (Figure 5). The projected lifetime incidence of melanoma in the United States has increased to between 1% and 2%.[7] Patient prognosis and staging are based on depth of tumor invasion (Breslow depth), tumor ulceration, nodal involvement, and the presence of local, in-transit, and systemic metastases.[8]
Current guidelines for the surgical management of primary cutaneous melanoma are based on standard surgical excision, with increasing surgical margins depending on the depth of tumor invasion.[9] Despite these recommendations, the potential therapeutic advantage of Mohs micrographic surgery to detect subclinical extensions of neoplastic cells missed by routine breadloaf sectioning and tissue conservation has prompted several clinicians to investigate the utility of this modality in the treatment of melanoma.
FIGURE 4
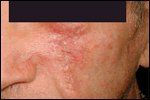
After Surgery and Reconstruction
The first case series of melanoma patients treated with chemosurgery was reported by Frederic Mohs himself in 1950.[10] His initial findings revealed local recurrence and survival rates comparable to those of traditional excisional surgery. Since that time, several other case series have documented similar or improved recurrence and survival rates with the use of Mohs micrographic surgery.[ 11-13] In the most recent and largest of these, Bricca et al reported a cohort of 625 consecutive patients with melanoma or melanoma in situ of the head and neck region who were treated with Mohs micrographic surgery.[13] After a mean follow-up of 58 months, their results demonstrated local recurrence rates, metastasis rates, and disease-specific survival rates that were comparable to or better than those of historical controls for every Breslow stratification. In addition to increased tumor clearance, they found that this technique also provided tissue conservation in the majority of cases, as 6-mm margins were sufficient to clear 83% of melanomas.
FIGURE 5
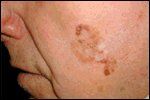
Melanoma In Situ
• Controversies in Use for Melanoma-Despite recent encouraging results, several arguments remain against the use of Mohs micrographic surgery in the treatment of melanoma.
The first of these points concerns difficulties inherent to the histopathologic diagnosis of melanoma, and in particular melanoma in situ, on frozen tissue sections as compared to traditional paraffin-embedded sections. Differentiating the neoplastic cells of melanoma in situ from the background melanocytic hyperplasia of chronically actinic-damaged skin on paraffin sections can be challenging for the dermatopathologist. This task becomes more difficult when frozen sections are utilized, and has led some authors to propose that frozen sections are unreliable in the detection of margins of melanoma in situ.[14]
However, in a review of 221 specimens, Zitelli et al found frozen sections to have a sensitivity of 100% and specificity of 90% in the diagnosis of melanoma as compared with routine paraffin sections.[15] This improvement in diagnosis is the result of advances in immunohistochemical staining, as melanocytes, whether present individually or in small clusters, are now more readily identified on frozen sections. Of these immunostains, melanoma antigen recognized by T cells (MART-1) has proven to be the most sensitive except in the case of desmoplastic melanoma, in which the use of S-100 is indicated.[16,17]
A second theoretical argument against the use of Mohs micrographic surgery for melanoma is the belief that by taking wide margins around the tumor, the primary tumor as well as small local micrometastases are being excised, resulting in improved local recurrence and long-term survival rates vs narrow but complete excision of the primary tumor alone. This theory has not been substantiated by the data on excision of melanoma, which through the years has resulted in a reduction of the recommended surgical margins for melanoma.[ 8,9] It is also contradicted by studies displaying improved local recurrence, metastasis, and survival rates for Mohs micrographic surgery in the treatment of melanoma as compared to historical controls treated with conventional surgical resection.[11-13]
Merkel Cell Carcinoma
Merkel cell carcinoma is an aggressive neoplasm of the skin that was originally described by Toker in 1972 as trabecular cell carcinoma.[18] It is believed to be derived from the Merkel cell, which is a cell of neuroendocrine origin located in the basal layer of the epidermis involved in mediating the sensation of pressure. Merkel cell carcinoma is a rare neoplasm with an estimated annual incidence of 0.23 per 100,000 in Caucasians. It is primarily a tumor of older individuals, with an average age at presentation of 69 years and less than 5% of tumors occurring in patients younger than age 50. There is a slightly increased ratio of tumors in males compared to females (2.3:1).[19]
Merkel cell carcinoma typically presents as an erythematous-to-violaceous subcutaneous nodule or plaque. The overlying skin usually remains intact but may exhibit central ulceration. It occurs predominantly in sun-exposed areas of skin, with 50% of tumors arising on the head and neck and 40% on the extremities.[19] It is almost exclusively a disease of fair-skinned individuals. This, in combination with its usual anatomic presentation, has led investigators to presume that ultraviolet radiation is a primary factor in its etiology. Similar to SCC, Merkel cell carcinoma also appears to occur with increased frequency in patients who have undergone solid organ transplantation and those with other forms of immunosuppression.
Merkel cell carcinoma exhibits an aggressive biologic behavior with the potential for rapid spread through either lymphatic or hematogenous routes. Approximately 30% of patients have regional nodal mestastases at the time of presentation. Reported 5-year survival rates range from 30% to 81%. Due to the rarity of this tumor, no prospective randomized trials exist to guide surgical management. Currently, the most common approach to the patient with stage I disease (ie, tumor clinically localized to the skin) is wide local excision of the primary tumor with 2- to 3-cm margins of clinically uninvolved skin, with or without adjuvant radiation. Studies employing this approach have produced local recurrence rates ranging from 13% to 39%.[20,21] Complete resection of the primary tumor is of paramount importance, as two-thirds of those who develop local recurrences ultimately die of their disease.[21]
With complete examination of the surgical margin, Mohs surgery was recognized as a possible alternative to wide local excision for optimizing resection of the primary tumor and minimizing local recurrence. The first series of patients with Merkel cell carcinoma treated with Mohs micrographic surgery was reported in 1997 by O'Connor et al.[22] This retrospective study analyzed rates of local recurrence and regional metastasis in patients treated with either Mohs surgery (n = 12) or wide local excision (n = 41). Local recurrence was identified in 8.3% of patients treated with Mohs surgery vs 31.7% of those with wide local excision. Regional metastases developed in one-third of the Mohs surgery group and 48.8% of the wide local excision group.
Boyer et al reviewed 45 cases of Merkel cell carcinoma treated with Mohs surgery; 20 of the patients in this series also received adjuvant radiation therapy.[23] A local recurrence, which the authors defined to include both marginal recurrence and in-transit metastases, was observed in 8.8% of patients-16% of those treated with Mohs alone but none of those treated with Mohs surgery plus radiation therapy. The overall 5-year survival estimate for both groups was 79% and 80%, respectively.
Thus, initial results of the treat treatment of Merkel cell carcinoma with Mohs surgery appear to demonstrate local recurrence and survival rates comparable to or better than those of wide local excision. That said, the experience with Mohs surgery in the management of Merkel cell carcinoma remains limited.
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans is an uncommon mesenchymal neoplasm that originates in the dermis. The tumor is characterized by a locally aggressive growth pattern in which ramifications of spindle cells infiltrate the dermis and surrounding tissues. With time, involvement of the subcutaneous fat, muscle, cartilage, and bone may occur.[24] The potential for hematogenous or lymphatic dissemination does exist but is rare. Clinically, the tumor typically presents as an erythematous-to-violaceous nodule or plaque with poorly circumscribed clinical margins. It occurs primarily on the trunk and proximal extremities of individuals in their 30s and 40s.
With reported local recurrence rates of 30% to 50% for simple excision and 20% for wide local excision,[ 24] Mohs surgery has emerged as the treatment of choice for dermatofibrosarcoma protuberans. In a series of 29 cases with more than 5 years of follow-up, Snow et al experienced no recurrences in primary and recurrent tumors treated with Mohs surgery.[25] Their review of 136 cases in the literature with similar follow-up documented 9 cases of recurrence, for a local recurrence rate of 6.6%. In a study of 22 patients with dermatofibrosarcoma protuberans treated with a modified Mohs technique utilizing rush paraffin sections, no recurrences were observed.[26]
Despite the absence of randomized controlled trials, Mohs surgery appears to offer lower recurrence rates than conventional surgical excision for the treatment of dermatofibrosarcoma protuberans. Experience in the treatment of other spindled cell tumors of the skin with Mohs surgery is more limited. However, Heuther et al reported local recurrence rates of 6.9% for atypical fibroxanthoma, 43% for malignant fibrous histiocytoma, and 14% for leiomyosarcoma.[27] The role of Mohs surgery in the management of these other spindled neoplasms remains unclear and will become better defined with increased experience.
Extramammary Paget's Disease
Extramammary Paget's disease is a rare cutaneous adenocarcinoma believed to be derived from apocrine cells. Cases primarily occur in regions of high apocrine gland distribution, namely the vulva, penis, scrotum, perianal region, and less frequently, the axilla. The precise histogenesis of this tumor is unknown, but 10% to 20% of cases have been observed in association with an internal malignancy, usually of the gastrointestinal or genitourinary system.[28,29]
Extramammary Paget's disease typically presents as an erythematous macerated patch of the anogenital region. A delay in diagnosis of 5 to 10 years is not uncommon, as the clinical presentation may be mistaken for other dermatoses such as tinea cruris, erythrasma, inverse psoriasis, or contact dermatitis.[30] The growth of this neoplasm is usually intraepidermal in nature with involvement of adjacent adnexal structures; however, dermal invasive disease with subsequent regional and systemic metastases does occur in up to 10% of cases.[29] The prognosis is poor once dissemination has begun.
Surgical management of the primary tumor of extramammary Paget's disease is confounded by the neoplasm's indistinct clinical margins and propensity for extensive subclinical spread. The literature reports local recurrence rates of 33% to 60% for standard surgical excision with frozen and/or fixed margin control.[30-34] In addition, for cases treated with wide local excision, reported local recurrence rates remain high at 20% to 50%.[31-34]
These high local recurrence rates, in addition to the known tendency of extramammary Paget's disease for broad subclinical extension, prompted several investigators to examine the use of Mohs surgery as an alternative treatment. The largest reported series of extramammary Paget's disease treated with Mohs surgery consisted of 27 cases (19 primary tumors, 8 recurrent tumors) with an average follow-up of 76 months.[35] Their results revealed a local recurrence rate of 16% for primary extramammary Paget's disease and 50% for recurrent tumors (23% local recurrence rate for combined primary and recurrent tumors). Similar results, with a local recurrence rate of 23%, were reported by Coldiron et al in a survey of 48 cases of primary and recurrent extramammary Paget's disease treated with Mohs surgery.[30]
In a retrospective review, O'Connor et al examined local recurrence rates in 83 patients treated with wide local excision as compared to 12 patients treated with Mohs micrographic surgery.[ 29] Locally recurrent disease was observed in 22% of the wide local excision group and 8% of the Mohs surgery group; however, it should be noted that the two groups had significantly different mean follow-up periods (65 vs 24 months).
Although the existing data regarding the use of Mohs surgery in the treatment of extramammary Paget's disease are limited, such results appear favorable in comparison with those of wide local excision. Regardless of whether Mohs surgery or wide local excision is utilized, several adjunct techniques have been identified to optimize complete surgical excision of extramammary Paget's disease. Identification of subclinical spread of tumor may be aided by the use of peripheral scouting biopsies, topical imiquimod (Aldara) or fluorouracil cream,[36] and photodynamic therapy.[29] Histopathologic identification of residual tumor is aided by the use of immunohistochemical staining with cytokeratin-7[37] and carcinoembryonic antigen.[38]
Microcystic Adnexal Carcinoma
Microcystic adnexal carcinoma is a locally aggressive neoplasm that was first described in 1982.[39] Although the tumor does not appear to have the potential for metastasis, it is characterized by extensive subclinical spread of the malignancy beyond the identifiable tumor margins. Perineural invasion of tumor is not uncommon in the dermis, and invasion of deeper local structures such as the subcutaneous fat, muscle, and cartilage has been reported.[40]
FIGURE 6
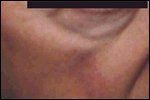
Microcystic Adnexal Carcinoma
Microcystic adnexal carcinoma typically presents as a slightly indurated, flesh-colored plaque of the face (Figure 6). It occurs primarily in whites, but a single case has been reported in an African-American male.[41] There appears to be a modestly increased incidence in females over males. It has been observed in all age groups, but highest incidence has occurred in patients in their 7th decade.[42] There is often a prior history of x-ray irradiation of the site for the treatment of acne.[43]
Given the indistinct nature of the clinical borders of microcystic adnexal carcinoma, Mohs surgery would appear ideal for surgical management of the tumor. Recurrence rates with conventional surgical excision have been estimated at 47%, based on review of the literature.[43] In a series of 11 cases of microcystic adnexal carcinoma treated with Mohs surgery, Friedman et al observed no recurrences over a mean follow-up of 5 years.[43] In a review of the Australian experience of the treatment of microcystic adnexal carcinoma with Mohs surgery, Lebovitch at al reported a 5% recurrence rate in the 20 patients with at least 5 years follow-up.[44] The largest available series of patients with microcystic adnexal carcinoma treated with either Mohs surgery or traditional excision found a local recurrence rate of 12% for the Mohs group and 17% for the excision group.[42] In addition, 30% of the group treated with standard excision required multiple additional procedures due to persistence of disease after initial excision.
The available data on the optimal therapy for microcystic adnexal carcinoma is sparse, and no prospective randomized controls exist due to the rarity of this tumor. Mohs micrographic surgery does appear to offer an advantage over traditional excision in the treatment of this locally aggressive neoplasm. The major challenge in the management of microcystic adnexal carcinoma with Mohs surgery is the accurate histopathologic interpretation of frozen sections of this tumor. This task is highly dependent upon both the quality of the sections produced by the histotechnician and the experience of the Mohs surgeon. Adjunctive measures to increase the sensitivity of this process include the use of immunohistochemical staining[45] and a modified Mohs technique in which rush paraffin sections are used.
Conclusions
Fundamentally, Mohs micrographic surgery differs from conventional surgical excision by its ability to provide complete histologic examination of surgical margins. This technique allows the Mohs surgeon to maximize normal tissue conservation through narrower surgical margins while achieving the highest cure rates for the treatment of the most common cutaneous neoplasms.
Mohs surgery has been wellestablished as the gold standard for the treatment of BCCs and SCCs.[1-3] Preliminary reports suggest that it may play an equally important role in the management of several other cutaneous malignancies, as described above. Further experience will better define this role and provide additional insight into other uses.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Rowe DE, Carroll RJ, Day CL: Long-term recurrence rates in previously untreated basal cell carcinoma: Implications for patient follow-up. J Dermatol Surg Oncol 15:315-328, 1989.
2. Rowe DE, Carroll RJ, Day CL: Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol 15:424-431, 1989.
3. Rowe DE, Carroll RJ, Day CL: Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 26:976- 990, 1992.
4. Abide JA, Nahai F, Bennett RG: The meaning of surgical margins. Plast Reconstr Surg 73:492-497, 1984.
5. Brodland DG, Amonette R, Hanke CW, et al: The history and evolution of Mohs micrographic surgery. Dermatol Surg 26:303-307, 2000.
6. Tromovitch TA, Stegman SJ: Microscopically controlled excision of skin tumors. Arch Dermatol 110:231-232, 1974.
7. Jemal A, Murray T, Ward E, et al: Cancer statistics, 2005. Ca Cancer J Clin 55:10-30, 2005.
8. Balch CM, Buzaid AC, Soong SJ, et al: Final version of the American Joint Committee on Cancer Staging System for cutaneous melanoma. J Clin Oncol 19:3635-3648, 2001.
9. Sober AJ, Chuang TY, Duvic M, et al: Guidelines of care for primary cutaneous melanoma. J Am Acad Dermatol 45:578-586, 2001.
10. Mohs FE: Chemosurgical treatment of melanoma: A microscopically controlled method of excision. Arch Dermatol Syph 62:269-272, 1950.
11. Bienert TN, Trotter MJ, Arlette JP: Treatment of cutaneous melanoma of the face by Mohs micrographic surgery. J Cutan Med Surg 7:25- 30, 2003.
12. Zitelli JA, Mohs FE, Larson P, et al: Mohs micrographic surgery for melanoma. Dermatol Clin 7:833-843, 1989.
13. Bricca GM, Brodland DG, Ren D, et al: Cutaneous head and neck melanoma treated with Mohs micrographic surgery. J Am Acad Dermatol 52:92-100, 2005.
14. Barlow RJ, White CR, Swanson NA: Mohs’ micrographic surgery using frozen sections alone may be unsuitable for detecting single atypical melanocytes at the margins of melanoma in situ. Br J Dermatol 146:290-294, 2002.
15. Zitelli, JA, Moy RL, Abell EA: The reliability of frozen sections for the evaluation surgical margins of melanoma. J Am Acad Dermatol 24:102-106, 1991.
16. Bricca GM, Brodland DG, Zitelli JA: Immunostaining melanoma frozen sections: The 1 hour protocol. Derm Surg 30:403-408, 2004.
17. Kelley LC, Starkus L: Immunohistochemical staining of lentigo maligna during Mohs micrographic surgery using MART-1. J Am Acad Dermatol 46:78-84, 2002.
18. Toker C: Trabecular carcinoma of the skin. Arch Dermatol 105:107-110, 1972.
19. Miller RW, Rabkin CS: Merkel cell carcinoma and melanoma: Etiological similarities and differences. Cancer Epidemiol Biomarkers Prev 8:153-158, 1999.
20. Yiengpruksawan A, Coit DG, Thaler HT, et al: Merkel cell carcinoma. Prognosis and management. Arch Surg 126:1514-1519, 1991.
21. Shaw JH, Rumball E: Merkel cell tumour: Clinical behaviour and treatment. Br J Surg 78:138-142, 1991.
22. O’Connor WJ, Roenigk RK, Brodland DG: Merkel cell carcinoma: Comparison of Mohs micrographic surgery and wide excision in 86 patients. Dermatol Surg 23:929-933, 1997.
23. Boyer JD, Zitelli JA, Brodland DG, et al: Local control of primary Merkel cell carcinoma: Review of 45 cases treated with Mohs micrographic surgery with and without adjuvant radiation. J Am Acad Dermatol 47:885-892, 2002.
24. Gloster HM, Harris KR, Roenigk RK: A comparison between Mohs micrographic surgery and wide surgical excision for the treatment of dermatofibrosarcoma protuberans. J Am Acad Dermatol 35:82-87, 1996.
25. Snow SN, Gordon EM, Larson PO, et al: Dermatofibrosarcoma protuberans: A report on 29 patients treated by Mohs micrographic surgery with long-term follow-up and review of the literature. Cancer 101:28-38, 2004.
26. Wacker J, Khan-Durani B, Hartschuh W: Modified Mohs micrographic surgery in the therapy of dermatofibrosarcoma protuberans: Analysis of 22 patients. Ann Surg Oncol 11:438- 444, 2004.
27. Huether MJ, Zitelli JA, Brodland DG: Mohs micrographic surgery for the treatment of spindle cell tumors of the skin. J Am Acad Dermatol 44:656-659, 2001.
28. Chanda JJ: Extramammary Paget’s disease: Prognosis and relationship to internal malignancy. J Am Acad Dermatol 13:1009-1014, 1985.
29. O’Connor WJ, Lim KK, Zalla MJ, et al: Comparison of Mohs micrographic surgery and wide excision for extramammary Paget’s disease. Dermatol Surg 29:723-727, 2003.
30. Coldiron BM, Goldsmith BA, Robinson JK: Surgical treatment of extramammary Paget’s disease: A report of six cases and a reexamination of Mohs micrographic surgery compared with conventional surgical excision. Cancer 67:933-938, 1991.
31. Marchesa P, Fazio VW, Oliart S, et al: Long-term outcome of patients with perianal Paget’s disease. Ann Surg Oncol 4:475-480, 1997.
32. Zollo JD, Zeitouni NC: The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br J Dermatol 142:59-65, 2000.
33. McCarter MD, Quan SH, Busam K, et al: Long-term outcome of perianal Paget’s disease. Dis Colon Rectum 46:612-616, 2003.
34. Sarmiento JM, Wolff BG, Burgart LJ, et al: Paget’s disease of the perianal region: An aggressive disease? Dis Colon Rectum 40:1187- 1194, 1997.
35. Hendi A, Brodland DG, Zitelli JA: Extramammary Paget’s disease: Surgical treatment with Mohs micrographic surgery. J Am Acad Dermatol 51:767-773, 2004.
36. Eliezri YD, Silvers DN, Horan DB: Role of preoperative topical 5-flourouracil in preparation for Mohs micrographic surgery of extramammary Paget’s disease. J Am Acad Dermatol 17:497-505, 1987.
37. Smith KJ, Tuur S, Corvette D, et al: Cytokeratin 7 staining in mammary and extramammary Paget’s disease. Mod Pathol 10:1069-1074, 1997.
38. Harris DW, Kist DA, Bloom K, et al: Rapid staining with carcinoembryonic antigen aids limited excision of extramammary Paget’s disease treated by Mohs surgery. J Dermatol Surg Oncol 20:260-264, 1994.
39. Goldstein DJ, Barr RJ, Cruz DJ: Microcystic adnexal carcinoma: A distinct clinicopathologic entity. Cancer 50:566-572, 1982.
40. Birkby CS, Argenyl ZB, Whitaker DC: Microcystic adnexal carcinoma with mandibular invasion and bone marrow replacement. J Dermatol Surg Oncol 15:308-312, 1989.
41. Park JY, Parry EL: Microcystic adnexal carcinoma: First reported case in a black patient. Dermatol Surg 24:905-907, 1998.
42. Chiller K, Passaro D, Scheuller M, et al: Microcystic adnexal carcinoma: Forty-eight cases, their treatment and their outcome. Arch Dermatol 136:1355-1359, 2000.
43. Friedman PM, Friedman RH, Jiang B, et al: Microcystic adnexal carcinoma: Collabora- tive series review and update. J Am Acad Dermatol 41:225-231, 1999.
44. Lebovitch I, Huilgol, Selva D: Microcystic adnexal carcinoma: Treatment with Mohs micrographic surgery. J Am Acad Dermatol 52:295- 300, 2005.
45. Wick MR, Cooper PH, Swanson PE, et al: Microcystic adnexal carcinoma: An immunohistochemical comparison with other cutaneous appendage tumors. Arch Dermatol 126:189-194, 1990.