Bone-Modifying Agents as Adjuvant Therapy for Early-Stage Breast Cancer
In this review we describe the current evidence for use of bisphosphonates as part of the adjuvant treatment of patients with early-stage breast cancer.
The development of effective systemic therapies to reduce the risk of disease recurrence or metastases in early-stage breast cancer remains an important challenge. The use of bone-modifying agents (BMAs), including the bisphosphonates (BPs) and the monoclonal antibody denosumab (Xgeva), is well established for metastatic bone disease. In the adjuvant setting, some studies have shown provocative findings with some of these agents for the prevention of future breast cancer–related events, with improved survival in some subgroups. The most compelling results have been seen with clodronate and zoledronic acid. In this review we describe the current evidence for use of BPs as part of the adjuvant treatment of patients with early-stage breast cancer.
Introduction
The bisphosphonate (BP) drugs have a long established role in the management of osteoporosis, hypercalcemia of malignancy, and bone metastases from solid tumors and multiple myeloma. In the breast cancer population, zoledronic acid (ZA) and pamidronate have been widely used for over a decade in patients with bone metastases as an adjunct to radiotherapy, hormonal therapy, and chemotherapy, based on trials that have shown a reduction in skeletal-related events (SREs), including fractures, spinal cord compression, and worsening pain necessitating radiotherapy.[1] In addition, these agents are being suggested for premenopausal patients with bone loss from premature ovarian failure due to chemotherapy and in postmenopausal patients treated with aromatase inhibitors (AIs).[2] There is increasing interest in using these drugs to reduce the risk of breast cancer metastases, both bone and non-bone, independent of the established uses for bone-related indications; this is in light of a plausible mechanism of action based on preclinical evidence, some compelling observational data, and results from several interventional trials suggesting a role in some subpopulations of breast cancer patients. However, trial results with BPs are conflicting, and currently none of these agents have been designated by the US Food and Drug Administration (FDA) as being indicated for reducing breast cancer recurrence or improving disease-free survival (DFS) and overall survival (OS), so any use for this purpose in the US must be considered off-label and experimental.
This manuscript will review the preclinical and clinical data for the use of bone-modifying agents (BMAs) in the adjuvant treatment of early breast cancer. We will examine clinical trial results for these agents and will address issues of toxicity and cost-effectiveness. We will attempt to offer a perspective on their use in the absence of existing guidelines for the primary purpose of reducing breast cancer recurrence.
Bone-Modifying Agents and Early Breast Cancer: Preclinical Observations
In normal bone, particularly in young adults, there is a balance between osteoclastic bone resorption and osteoblastic bone formation. This can be altered by the presence of tumor cells in bone, which accelerate resorption by promoting osteoclast formation and activity through the release of tumor cell–derived factors, such as parathyroid hormone–related peptide (PTHrP), prostaglandin-E, and bone sialoprotein. PTHrP binds to receptors on osteoblasts, enhancing production of receptor activator of nuclear factor κβ ligand (RANKL). This pathway results in the differentiation of osteoclasts and their activation. On osteoclasts, RANKL binds to its receptor, RANK, promoting an increase in bone resorption.[3]
Osteoclast inhibition induced by bisphosphonates (BPs) can alter the bone microenvironment, making it less supportive of tumor cell proliferation, and preclinical data suggest that BPs can reduce the likelihood of developing bone metastases.[4] The mechanisms of antitumor activity of BPs have not been fully elucidated, but it is proposed that these drugs exert both indirect and direct antitumor effects. One potential indirect mechanism of action would be inhibition of bone resorption, in which BPs reduce the release of cytokines and other growth factors. This makes the bone environment less hospitable for tumor cell migration, decreasing the likelihood of cell adhesion and proliferation. Another indirect antitumor effect is the induction of a T cell–mediated immune response, in which BPs may inhibit angiogenesis by activating T-effector cells producing interferon-gamma.[3]
A potential direct mechanism of BPs is the inhibition of angiogenesis, as shown in particular with ZA.[5,6] The BPs also decrease the adhesion, invasion, and proliferation of tumor cells. Moreover, they may have synergistic antitumor activity with cytotoxic agents, mainly with taxanes and anthracyclines.[3] For instance, the combination of ZA and doxorubicin produced synergistic antitumor activity in a primary breast cancer murine model without extraskeletal disease.[7] Additionally, antitumor effects appear to be dose-dependent, and in some in vitro studies BPs may directly and dose-dependently induce apoptosis in breast cancer cell lines.[8-11]
The hypothesis of the “seed and soil” described by Paget[12] may further elucidate the antitumor properties of BPs. The “seed” represents the tumor cell and the “soil” represents the bone marrow, which would have the ideal microenvironment to receive tumor cells. Supportive stromal cells, growth factors, and other components of the extracellular matrix, including the vasculature, make the bone marrow microenvironment a favorable milieu for cancer cell proliferation and propagation.[13] Some preclinical evidence also suggests that the bone marrow is a “premetastatic niche,” and the engraftment of tumor cells onto a favorable bone marrow microenvironment would begin the evolution of micrometastasis, to macrometastasis, to distant, non-bone metastasis.[14] Thus, early intervention such as the use of BPs in the adjuvant setting may interfere with the formation of this premetastatic niche.
Bone-Modifying Agents and Early Breast Cancer: Clinical Observations
Clodronate
Studies of clodronate as adjuvant therapy have reported mixed results, with variable effects on DFS and OS. In a prospective, non–placebo-controlled study by Diel et al,[15] patients with primary breast cancer stage T1-T4,N0-N2, who had immunocytochemical evidence of one or more tumor cells in a bone marrow aspirate, were randomly assigned either to treatment with clodronate at 1600 mg orally per day for 2 years or to standard follow-up care. A total of 302 patients were accrued between February 1990 and April 1997. The majority were stage T2, node-positive, estrogen receptor (ER)–positive, and postmenopausal. A total of 246 (81%) received adjuvant systemic treatment. During the median observation period of 36 months, the Kaplan–Meier curves showed significant differences between the two groups in metastasis-free survival (P < .001) and OS (P = .001), in favor of the clodronate group. The differences were also significant considering the proportion of patients developing bony metastases (P = .003) and visceral metastases (P = .003). After 103 months of follow-up, the significant improvement in OS was maintained in the clodronate group (P = .049); however, a significant difference in the incidence of bony(P = .770) and visceral metastasis (P = .222) between the clodronate and control groups was no longer seen.[16]
Powles et al[17] conducted a double-blind, multicenter trial that accrued 1069 patients with operable breast cancer given oral clodronate, 1600 mg daily, or placebo for 2 years. Mortality was significantly reduced for patients randomized to clodronate (hazard ratio [HR] = 0.77; P = .047). Five-year OS was 82.9% for clodronate and 79.3% for placebo (P = .047). This trial showed a decrease in the subsequent occurrence of bone metastases, but this reduction was only significant during the medication period (P = .016). In contrast to the Diel trial, there was no reduction in the incidence of visceral metastasis in the clodronate-treated group.
However, a study by Saarto et al[18] did not find a similar benefit for clodronate. In this trial, 299 pre- and postmenopausal women with T1-T3,N1-N2 breast cancer were randomized to clodronate for 3 years or control. Unlike in the other studies, the development of visceral recurrence was significantly higher in the clodronate group compared with controls: 43% vs 25% (P = .0007). The OS and DFS rates were also significantly lower in the clodronate group compared with controls (OS, 70% vs 83%, P = .009; DFS, 56% vs 71%, P = .007, respectively). The negative impact of clodronate was more prominent in patients with more advanced disease (large tumors, higher number of positive nodes, ER-negative disease). Especially in ER-negative patients, the DFS difference was large (29% in favor of the control group). In a multivariate analysis, after adjusting for progesterone receptor status, the adverse effect of clodronate on OS lost its significance, but there was still a significant adverse effect on DFS. These negative results may be explained in part by an imbalance between the treatment arms, with more hormone receptor–negative patients in the clodronate arm. After 10 years of follow-up, however, there was no significant compromise in OS (54% for the clodronate group vs 62% for controls, P = .13), but DFS remained significantly lower in the clodronate group (45% with clodronate vs 58% for controls, P = .01). This difference in DFS was especially observed in ER-negative patients (25% vs 58%, P = .004).[19]
In 2007 a meta-analysis[20] of the three studies described above demonstrated no statistically significant difference in OS between patients treated with adjuvant clodronate and those receiving no treatment (HR = 0.75; 95% confidence interval [CI], 0.31–1.82). Similarly, there was no difference in time to appearance of bone metastases (HR = 0.68; 95% CI, 0.38–1.23) or delay in the occurrence of visceral metastases (HR = 0.89; 95% CI, 0.40–1.98).
TABLE 1
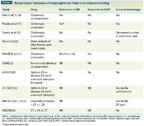
Breast Cancer Outcomes of Bisphosphonate Trials in the Adjuvant Setting
The final analysis of National Surgical Adjuvant Breast and Bowel Project (NSABP) B-34, a prospective, randomized trial assessing 3323 patients with stage I to III breast cancer who received oral clodronate at 1600 mg daily for 3 years compared with placebo, given alone or in addition to adjuvant chemotherapy or hormone therapy, was presented at the 2011 San Antonio Breast Cancer Symposium.[21] More than two-thirds of the patients were older than 50 years of age and had ER-positive tumors. The median follow-up was 8.41 years, and the long-term results showed no difference in DFS at any point in time (HR = 0.913; P = .266). Treatment compliance was poor, with only 42% of patients completing assigned study therapy. The greatest drop in compliance occurred during the first 6 months of the study when patients received concurrent chemotherapy, which may partially explain the negative result. Examination of the secondary endpoints did show a significant difference in favor of clodronate with respect to the non–bone- metastasis-free interval (NBMFI) (HR = 0.743; P = .046). In addition, prespecified subgroup analyses that included stratification by patient age at study entry showed that women older than 50 consistently derived more benefit from clodronate than did younger women. DFS remained similar, but analysis of secondary endpoints showed clear separation between treatment groups for recurrence-free interval (HR = 0.76; P = .05), bone metastasis–free interval (HR = 0.61; P = .024), and NBMFI (HR = 0.63; P = .015), all favoring clodronate for older patients. A post-hoc analysis with further age stratification showed that patients older than 60 derived the most benefit from clodronate, including an almost 60% reduction in skeletal metastases and a 40% to 50% reduction in nonskeletal metastases.[21] Therefore, the findings of the B-34 trial were consistent with those observed in older postmenopausal women in other BP studies (Tables 1–3).
Ibandronate
TABLE 2
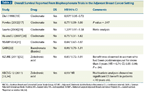
Overall Survival Reported From Bisphosphonate Trials in the Adjuvant Breast Cancer Setting
In the German Adjuvant Intergroup Node-Positive (GAIN) study, investigators found a similar lack of effect of ibandronate (Boniva) on DFS in patients with node-positive, early breast cancer treated with dose-dense chemotherapy.[22] In this trial, 3023 patients were randomized to a standard regimen of epirubicin, paclitaxel, and cyclophosphamide or to lower doses of the same three drugs plus capecitabine (Xeloda), and were further randomized 2:1 to 2 years of treatment with ibandronate at 50 mg orally daily or to observation. The primary endpoint was DFS, with separate analyses for the chemotherapy regimens and ibandronate vs observation. Although the protocol specified 5 years of follow-up, an interim analysis after 50% of the required events had occurred showed that the futility boundary had been crossed with respect to the comparison of ibandronate vs observation. Follow-up continues for the two chemotherapy regimens. At 39 months, patients in the ibandronate arm had a 3-year DFS of 87.6% vs 87.2% for the observation arm (HR = 1.059; 95% CI, 0.861–1.301). Analysis of 3-year OS (secondary endpoint) showed a similar lack of difference (HR = 0.961; 95% CI, 0.705–1.31): 94.7% for ibandronate and 94.1% for observation.
Pamidronate
TABLE 3
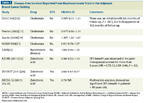
Disease-Free Survival Reported From Bisphosphonate Trials in the Adjuvant Breast Cancer Setting
Results from two nonrandomized trials, both presented as abstracts, suggest that adjuvant pamidronate may lead to a significant reduction in bone metastases. Kokufu et al developed a trial of 90 breast cancer patients with four or more positive nodes who were assigned by patient preference to receive IV pamidronate (at 45 mg for 4 cycles, q 2 wks) or to serve as controls. After a median follow-up of 5.4 years, the incidence of bone metastases was significantly lower in the pamidronate arm (12.1% vs 38.6%, P = .008), with no difference in OS.[23]
Jung et al analyzed data on 429 stage I to III patients treated over a 4-year period with standard local and systemic therapy, and about 60% received adjuvant pamidronate (15 mg IV q 4 wks and 100 mg orally daily during adjuvant chemotherapy). The patients were perimenopausal, and the median follow-up was 3.5 years. The incidence of bone metastases was significantly lower in the pamidronate-treated patients (2.3% vs 8.7%), but there was no difference in DFS or OS.[24]
Zoledronic acid
The Zometa-Femara Adjuvant Synergy Trials (Z-FAST and ZO-FAST) are the first studies to evaluate an IV BP (ZA) for the prevention of bone loss in postmenopausal women receiving an adjuvant AI (letrozole [Femara]).[25,26] The design of both studies was similar, with differences in the site of development (Z-FAST in the US and ZO-FAST in Europe). Postmenopausal women with early ER-positive breast cancer receiving adjuvant letrozole were randomized to ZA for 5 years, either upfront (4 mg every 6 months) or delayed-start (initiated with a decrease in T score to less than −2 or the occurrence of a clinical nontraumatic fracture). The primary outcome was the change in lumbar spine (LS) bone mineral density (BMD) at month 12.[27] Upfront ZA progressively increased LS BMD throughout these studies; conversely, the delayed group experienced significant decreases in LS BMD at all time points. The final 5-year result of Z-FAST showed the adjusted mean difference in LS BMD between the upfront and delayed-start groups was 8.9% (P < .0001).[28] In the ZO-FAST trial, at 36 months of follow-up, mean change in the LS BMD was +4.39% for immediate vs −4.9% for delayed ZA (P < .0001). Between-group differences were 5.27% at 12 months, 7.94% at 24 months, and 9.29% at 36 months (P < .0001 for all).[29]
Moreover, both trials used time to disease recurrence as a secondary endpoint. Although a pooled analysis at 12 months of follow-up suggested that upfront ZA resulted in fewer episodes of disease recurrence or death, the final Z-FAST analysis with 5 years of follow-up showed no difference in either.[28] A preliminary report of 60 months follow-up of the ZO-FAST study showed a 34% improvement in DFS with immediate treatment with ZA, when compared with delayed therapy. An exploratory analysis of survival outcomes stratifying by menopausal status at the time of diagnosis demonstrated that postmenopausal women who began immediate treatment with ZA had a 29% reduction in the risk of recurrence and a 35% improvement in OS.[30]
The Austrian Breast and Colorectal Cancer Study Group trial 12 (ABCSG-12) and the Adjuvant Zoledronic Acid to Reduce Recurrence (AZURE) trial both evaluated the adjuvant use of ZA in patients with early-stage breast cancer,[31,32] and for both, the primary endpoint was DFS. However, there are some important differences between these studies. The ABCSG-12 trial initially assigned 1803 premenopausal women who had undergone primary surgery for stage I to II hormone receptor–positive breast cancer, and who had fewer than 10 positive lymph nodes, to receive goserelin (Zoladex) plus tamoxifen or goserelin plus anastrozole (Arimidex), with or without ZA for 3 years. Preoperative, but not adjuvant, chemotherapy was allowed. Most of the patients were older than 40, with T1 tumors, and were node-negative. Only 10% received preoperative chemotherapy. Thus, this study included patients with an excellent prognosis.[31] After 48 and 62 months of follow-up, respectively, the addition of ZA to adjuvant endocrine therapy significantly improved DFS, the primary outcome.[31,33] The most recent results after 76 months of follow-up, reported at the San Antonio Breast Cancer Symposium in 2011, showed that patients receiving ZA had a 27% reduction in the risk of DFS events (HR = 0.73; P = .022) and a 41% reduction in the risk of death (HR = 0.59; P = .027) when compared with patients who did not receive ZA. Multivariate analysis showed a strong interaction between ZA and patient age, but it did not show any interactions between ZA and assigned hormonal therapy (anastrozole or tamoxifen) or any classic tumor parameter (tumor size, nodal status, grade, estrogen receptor status). Among patients over 40 years of age, ZA significantly reduced the risk of recurrence by 34% (HR = 0.66; P = .014) and the risk of death by 49% (HR = 0.51; P = .020), suggesting that maximal estrogen blockade was required for effect. However, there was no improvement in either DFS or OS among patients younger than 40.[34]
In the AZURE trial, 3360 patients with node-positive (N1) or a T3-T4 breast cancer were randomized to receive standard adjuvant systemic therapy with or without ZA. This study did not select patients by menopausal status or ER status; however, the majority were ER-positive (78%). Although the groups were well-distributed in terms of menopausal status, it is important to emphasize that about 30% of patients were classified as postmenopausal for more than 5 years. This trial showed no benefit in DFS among patients treated with ZA compared with those who did not receive it (HR = 0.98; CI, 0.85–1.13; P = .79). In a subgroup analysis of postmenopausal patients, the rates of invasive disease–free survival were 78.2% in the ZA group and 71% in the control group (HR = 0.75; CI, 0.59–0.96; P = .02). Additionally, for patients in whom menopause had occurred more than 5 years prior to study entry, the 5-year OS was 84.6% in the ZA group and 78.7% in the control group (HR = 0.74; CI, 0.55–0.98; P = .04), as compared with premenopausal patients or those who had undergone menopause less than 5 years prior.[32]
There are important differences between these two trials, which may explain the divergent results. First, in ABCSG-12, the ZA dose was initially 8 mg every 4 weeks and was reduced to 4 mg every 6 months after a protocol amendment in 2000. In the AZURE trial, ZA was administered as 4 mg every 3 to 4 weeks for 6 cycles and then every 3 months for 8 doses, followed by 5 cycles on a 6-month schedule for a total of 5 years. Second, the ABCSG-12 population was at low risk for recurrence, and all patients had received endocrine therapy, including goserelin, prior to the initiation of BP treatment, which resulted in rapid ovarian suppression. Patients who seemed to benefit the most from ZA in ABCSG-12 were over 40 years of age, and presumably perimenopausal. This result should be contrasted with the AZURE data, in which only patients with a postmenopausal status for more than 5 years had improved survival.
Denosumab
Binding RANKL can also result in osteoclast inhibition.[35] Denosumab (Xgeva), a monoclonal antibody to RANKL, has demonstrated efficacy in delaying or preventing skeletal-related events (SREs) in patients with bone metastases from breast cancer.[36] Denosumab (at 120 mg subcutaneously q 4 wks) is FDA-approved to reduce the risk of SREs in patients with bone metastases from solid tumors. Furthermore, denosumab (at 60 mg subcutaneously q 6 mos) has been FDA-approved for management of postmenopausal women with osteoporosis. Denosumab has also been evaluated in patients with breast and prostate cancer who are at risk for cancer therapy–induced bone loss caused by AIs or androgen-deprivation therapy,[37-39] and it is FDA-approved for that indication, marketed as Prolia.
In the adjuvant setting, there is currently only one phase III clinical trial (an industry-sponsored trial called D-CARE [Study of Denosumab as Adjuvant Treatment for Women With High Risk Early Breast Cancer Receiving Neoadjuvant or Adjuvant Therapy]) investigating the ability of denosumab to prolong bone metastasis–free survival (BMFS) and DFS. Eligible for inclusion in the trial are women with stage II to III breast cancer who are at high risk for recurrence and whose hormone and human epidermal growth factor receptor 2 (HER2) status is known (estimated sample size = 4500). Standard-of-care adjuvant or neoadjuvant chemotherapy, endocrine therapy, or HER2-targeted therapy must be planned. Patients are randomized 1:1 to receive denosumab at 120 mg or placebo monthly for 6 months, then q 3 mos for a total of 5 years. The primary endpoint is BMFS. Secondary endpoints include DFS and OS. The trial began enrolling patients in June 2010 and is expected to complete enrollment in October 2016.[40]
Bone-Modifying Agents: Toxicity and Cost-Effectiveness
Although toxicity is one concern regarding routine use of BMAs, these drugs are generally well tolerated. BPs have been associated with adverse events involving the upper gastrointestinal tract (oral formulation); an acute-phase response, characterized by fever and influenza-like symptoms occurring mainly after the first infusion of intravenous BPs in naive patients; hypocalcemia and secondary hyperparathyroidism; musculoskeletal pain; nonspecific conjunctivitis; osteonecrosis of the jaw (ONJ); and renal dysfunction, particularly with pamidronate and ZA.[41] Similarly, denosumab, while generally well tolerated,[42] can cause hypocalcemia, skin infections, ONJ, and suppression of bone turnover.[37]
ONJ is considered one of the most clinically important toxicities, and it was defined by a task force of the American Society for Bone and Mineral Research as “the presence of exposed bone in the maxillofacial region that did not heal within 8 weeks after identification by a health care provider.”[43] Risk factors include periodontal disease and dental procedures involving bone surgery.[44] Despite ONJ being a serious adverse event, the incidence of ONJ with BPs during adjuvant therapy appears to be low, reported as 0.24% in a meta-analysis[45] and as 0.7% in an AZURE sub-study.[46]
Issues of cost-effectiveness must also be considered when the optimal bone-protection strategy is recommended. At present, there are only reports of cost-effectiveness associated with bone events, not with breast outcomes. In 2009, El Ouagari and colleagues reported the cost-effectiveness of ZA plus endocrine therapy in the ABCSG-12 trial. The authors found that, from a Canadian healthcare perspective, adding ZA to goserelin plus tamoxifen/anastrozole in premenopausal women with ER-positive breast cancer is highly cost-effective.[47] Logman et al looked at the cost-effectiveness of ZA used for fracture prevention in postmenopausal women receiving AIs, modeled on interim BMD results from the ZO-FAST trial.[48] They concluded that it is cost-effective to initiate ZA after BMD levels have dropped below a T-score of −2 in postmenopausal ER-positive patients taking an AI whose initial BMD T-scores were −2 or higher.
Conclusion
Existing data do not clearly define the optimal and most appropriate adjuvant use of bisphosphonates, if any, to reduce breast cancer recurrence. Treatment guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network address use of BPs in the treatment of metastatic bone disease and other bone-related indications (eg, AI-induced osteoporosis), but are silent on the question of use in the adjuvant setting to reduce recurrence. Nonetheless, for many oncologists, particularly those in the U.S. who are comfortable with prescribing ZA for osteoporosis and for bone metastases, it is difficult to ignore the consistent results of ABCSG-12 and the subset analysis of AZURE, so ZA is often considered in this setting. It is reasonable to discuss these data sets with patients, recognizing that this use of ZA is off-label and may result in reimbursement challenges. Similarly, in countries where clodronate is available, its use may be considered in patients who match the subgroups that seemed to benefit in NSABP B-34.
Since no study to date has reported a DFS or OS benefit for denosumab in the adjuvant setting, its use for a non-bone endpoint should be avoided outside of a clinical trial. In addition, while the incidence of serious adverse events related to BPs seems quite low, studies in the metastatic setting have shown that ONJ incidence increases with duration of BP therapy, so the 0.7% incidence for ZA reported in AZURE may vary depending on the patient population, particularly since the true definition of ONJ and recommendations regarding reduction of predisposing factors continue to evolve. Also, it is important to note that ZA-associated first-dose infusion reactions, while usually self-limited, are occasionally noxious enough to discourage patients from subsequent doses.
If there are benefits to BPs in the adjuvant setting, they do not appear to extend to women younger than 40 years of age, as noted in AZURE, ABCSG-12, and NSABP-34. The greatest benefits may accrue to patients with a low-estrogen state: older postmenopausal women and premenopausal women receiving endocrine therapy that includes ovarian suppression. The Southwest Oncology Group trial S0307,[49] which randomized patients with stage I to III breast cancer to 3 years of either ZA, clodronate, or ibandronate, in addition to standard adjuvant therapy, closed to accrual in February 2010. This trial may further inform the discussion about the role of adjuvant BPs and their toxicities, when the results are available in a few years. Until then, the use of bisphosphonates will remain an area of controversy, and it may be best to reserve them for women with evidence of decreased BMD and a risk of fracture.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Acknowledgement:The authors would like to thank Dr. Vered Stearns for her invaluable assistance in reviewing this manuscript.
References:
References
1. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003; 98:1735-44.
2. Hadji P. Reducing the risk of bone loss associated with breast cancer treatment. Breast. 2007;16(Suppl 3):S10-5.
3. Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34:453-75.
4. Hall DG, Stoica G. Effect of the bisphosphonate risedronate on bone metastases in a rat mammary adenocarcinoma model system. J Bone Miner Res. 1994;9:221-30.
5. Bezzi M, Hasmim M, Bieler G, et al. Zoledronate sensitizes endothelial cells to tumor necrosis factor-induced programmed cell death: evidence for the suppression of sustained activation of focal adhesion kinase and protein kinase B/Akt. J Biol Chem. 2003;278:43603-14.
6. Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055-61.
7. Ottewell PD, Monkkonen H, Jones M, et al. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008;100:1167-78.
8. Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res. 2000;15:2211-21.
9. Senaratne SG, Pirianov G, Mansi JL, et al. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82:1459-68.
10. Verdijk R, Franke HR, Wolbers F, Vermes I. Differential effects of bisphosphonates on breast cancer cell lines. Cancer Lett. 2007;246:308-12.
11. Jagdev SP, Coleman RE, Shipman CM, et al. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br J Cancer. 2001;84:1126-34.
12. Paget S. The distribution of secondary growths in cancer of the breast. 1989. Cancer Metastasis Rev. 1989;8:98-101.
13. Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285-93.
14. Tabane K, Vorobiof DA. Bone targeted therapies in early breast cancer. Curr Treat Options Oncol. 2011;
12:412-23.
15. Diel IJ, Solomayer EF, Costa SD, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357-63.
16. Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol. 2008;19:2007-11.
17. Powles T, Paterson S, Kanis JA, et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol. 2002;
20:3219-24.
18. Saarto T, Blomqvist C, Virkkunen P, Elomaa I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. J Clin Oncol. 2001;19:10-7.
19. Saarto T, Vehmanen L, Virkkunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43:650-6.
20. Ha TC, Li H. Meta-analysis of clodronate and breast cancer survival. Br J Cancer. 2007;96:1796-801.
21. Paterson A, Anderson S, Lembersky B, et al. NSABP protocol B-34: a Clinical trial comparing adjuvant clodronate vs placebo in early stage breast cancer patients receiving systemic chemotherapy and/or tamoxifen or no therapy - final analysis. CTRC-AACR San Antonio Breast Cancer Symposium. 2011; abstr S2-3.
22. Mobus V, Diel I, Elling D, et al. GAIN study: a phase III trial to compare ETC vs EC-TX and ibandronate vs observation in patients with node-positive primary breast cancer - 1st Interim Efficacy Analysis. CTRC-AACR San Antonio Breast Cancer Symposium. 2011; abstr S2-4.
23. Kokufu I, Kohno N, Takao S, et al. Adjuvant pamidronate (PMT) therapy for the prevention of bone metastasis in breast cancer (BC) patients (pts) with four or more positive nodes. J Clin Oncol. 2004;22(14s):abstr 530.
24. Jung J, Hwang G, Lee Y, et al. Pamidronate as adjuvant treatment for prevention of bone metastasis in breast cancer. ASCO Annual Meeting. 2005; abstr 888.
25. Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007;25:829-36.
26. Bundred NJ, Campbell ID, Davidson N, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST study results. Cancer. 2008;
112:1001-10.
27. Brufsky A, Bundred N, Coleman R, et al. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13:503-14.
28. Brufsky AM, Harker WG, Beck JT, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118:1192-201.
29. Eidtmann H, de Boer R, Bundred N, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol. 2010;21:2188-94.
30. de Boer R, Bundred N, Eidtmann H, et al. Long-term survival outcomes among postmenopausal women with hormone receptor-positive early breast cancer receiving adjuvant letrozole and zoledronic acid: 5-year follow-up of ZO-FAST. CTRC-AACR San Antonio Breast Cancer Symposium 2011; abstr S1-3.
31. Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679-91.
32. Coleman RE, Marshall H, Cameron D, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365:1396-405.
33. Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631-41.
34. Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Long-term follow-up in ABCSG-12: significantly improved overall survival with adjuvant zoledronic acid in premenopausal patients with endocrine-receptor-positive early breast cancer. CTRC-AACR San Antonio Breast Cancer Symposium. 2011; abstr S1-2.
35. Lipton A, Uzzo R, Amato RJ, et al. The science and practice of bone health in oncology: managing bone loss and metastasis in patients with solid tumors. J Natl Compr Canc Netw. 2009;7(Suppl 7):S1-29.
36. Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132-9.
37. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-65.
38. Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875-82.
39. Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-55.
40. Goss P, Barrios C, Bell R, et al. Denosumab versus placebo as adjuvant treatment for women with early-stage breast cancer who are at high risk of disease recurrence (D-CARE): an international, randomized, double-blind, placebo-controlled phase III clinical trial. J Clin Oncol. 2012;30(suppl): abstr TPS670.
41. Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens). 2009;8:96-110.
42. Lipton A, Steger GG, Figueroa J, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431-7.
43. Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-91.
44. Ruggiero S, Gralow J, Marx RE, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2:7-14.
45. Mauri D, Valachis A, Polyzos IP, et al. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: a meta-analysis. Breast Cancer Res Treat. 2009;116:433-9.
46. Coleman R, Woodward E, Brown J, et al. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01-04) for women with stage II/III breast cancer. Breast Cancer Res Treat. 2011;127:429-38.
47. El-Ouagari K, Taneja C, Sofrygin O, et al. Cost-effectiveness of zoledronic acid plus endocrine therapy in premenopausal women with early breast cancer: Canadian perspective. ASCO Breast Cancer Symposium 2009; abstr 184.
48. Logman JF, Heeg BM, Botteman MF, et al. Economic evaluation of zoledronic acid for the prevention of osteoporotic fractures in postmenopausal women with early-stage breast cancer receiving aromatase inhibitors in the UK. Ann Oncol. 2010;21:1529-36.
49. Southwest Oncology Group: Zoledronate, clodronate, or ibandronate in treating women who have undergone surgery for stage I, stage II, or stage III breast cancer. S0307. [Updated May 19, 2012.] Available from: www.clinicaltrials.gov.
50. Wong MH, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2: CD003474.