Targeted Therapy: Its Status and Promise in Selected Solid Tumors Part I
We describe areas where major inroads were initially achieved by targeting angiogenesis and by unraveling pathways in the heterogeneous tumors of mesenchymal origin-spurred by the identification of c-Kit–activating mutations in GIST and the regressions that ensued when tumors harboring these mutations were exposed to the tyrosine kinase inhibitor imatinib (Gleevec).
“Targeted therapy” is becoming the centerpiece of current therapeutic strategies, and is often mentioned as the desirable direction for future progress. Why and how it is replacing past approaches in the management of solid tumors is the subject of this two-part overview. Here, in Part I, we describe areas where major inroads were initially achieved by targeting angiogenesis (central to the biology of renal cell carcinoma and hepatocellular cancer) and by unraveling pathways in the heterogeneous tumors of mesenchymal origin-spurred by the identification of c-Kit–activating mutations in gastrointestinal stromal tumors (GIST) and the regressions that ensued when tumors harboring these mutations were exposed to the tyrosine kinase inhibitor imatinib (Gleevec). More recently, the successes in the treatment of the notoriously refractory malignant melanoma via the targeting of a specific BRAF mutation and via immune activation represent an unprecedented achievement of this new therapeutic direction. For each cancer discussed in the first part of our overview, as well as in Part II, which will deal with more common cancers, we briefly cover the tumor biology, how targeting was achieved, the introduction of immune modulation or immune-conjugates, and the impact these therapies are having in the disease.
Introduction
Clinical cancer therapeutics has entered an era[1] in which advances by means of targeted therapies are leading to unprecedented successes-from the impact of imatinib (Gleevec) in chronic myeloid leukemia and gastrointestinal stromal tumor (GIST) in 2004, the striking cure rates achieved with adjuvant trastuzumab (Herceptin) for human epidermal growth factor receptor 2 (HER2)-amplified breast cancer in 2005, and convincing improvements via novel agents in outcomes for hepatocellular and renal cell cancers from 2006 on-to the groundbreaking inroads into successful palliation of aggressive phases of malignant melanoma[2] and new efficacious endocrine and immunologic control of prostate cancers in the past 2 years. Personalized medicine and the need for molecular profiling have justifiably become the Holy Grail for the development of future successful weapons against cancer.[3,4]
As has often been the case, hematologic malignancies have led the way in the introduction of this new generation of therapeutics-perhaps because of easy access to tumor samples for pharmacodynamics, and the narrow spectrum of cells of origin. The situation with solid tumors is considerably more complex, and the evolution of targeted therapies for these cancers is still in its infancy. Consequently, this review (which is being presented in two parts) consists of a perspective on current opportunities and the “work in progress” and is not a compendium of clinical trials with novel targeted agents still seeking an indication. In fact, we shall focus on integrating emerging treatments with therapeutic strategies that include both the older endocrine therapies (the “original targeted therapies”) against breast and prostate cancers, and the empirically-derived successful chemotherapies, such as platinums, that are used in gynecologic cancers. At present, and for some years to come, the treatment of most solid tumors will continue to rely on a patchwork of empirically derived and newly introduced molecularly targeted agents. However, prospective identification of targets in the clinic will not only illuminate their clinical significance, but will also further accelerate drug development-witness the way in which the introduction of gefitinib (Iressa) for lung cancer was followed in less than a decade by the identification of driver mutations, new targeted drugs, and the universal adoption of molecular profiling for treatment selection. (Lung cancer will be covered in Part II).
Questions addressed
There are four fundamental questions that need to be answered about any targeted therapy.
1. What is the underlying tumor biology that is being targeted?
2. How “targeted” are the so-called “targeted drugs”?
3. Is the targeted therapy also suitable for immunomodulation and/or immunoconjugation?
4. In what way does the targeted therapy constitute a meaningful improvement over chemotherapy?
For most of the tumors we will consider, all of these questions will be covered, even though the area of immunomodulation is currently in its infancy and experimental in most instances. However, the remarkable developments in immunomodulation in a number of diseases-for example, immunotherapy in melanoma (which is covered below), and immunoconjugates in certain forms of breast cancer (covered in Part II) and in Hodgkin lymphoma represent just the beginning of a role for “targeted” immunotherapy. In fact, great excitement has been generated by the identification of new T-cell and antigen-presenting−cell stimulatory pathways targeting Programmed Death (PD)-1 or its ligand, although it is too early in the development of these pathways to assess their impact on the treatment of specific cancers.
Major impact areas
Selected solid tumor groupings best illustrate the various stages in the evolution of this targeted therapy revolution. In this review-part I of the two-part series-we consider targeted therapies for renal cell carcinoma, hepatocellular carcinoma, melanoma, and sarcomas. We have chosen to focus on these cancers because the systemic treatment of these tumors is dominated by recently introduced molecularly targeted drugs. We describe how the tumor biology in each of these cancers has been largely responsible for the therapeutic advances.
Prostate cancer (although not covered in this series) affords another example of the connection between tumor biology and the development of targeted therapies. Endocrine therapy (which targets androgen-dependent growth) has been the hallmark of the treatment of prostate cancer for several decades. During the past 2 years, refinements in targeting have led to the introduction of abiraterone (Zytiga; a steroid synthetic pathway inhibitor) and enzalutamide (Xtandi [formerly MDV3100]; an androgen receptor inactivator). Treatment of this cancer is also relying on immune-stimulation and bone-seeking radio-immunoconjugates-with all of these advances opening up new therapeutic landscapes.
While Part I highlights the way in which targeted therapies have led to unprecedented therapeutic advances in previously erratically responsive areas, Part II will deal with areas in which targeted therapies have had a major impact in special subsets of patients (eg, in breast and lung cancers), and with those areas where we expect evolving integration into treatment as molecular pathways become better understood (eg, colorectal cancer and gynecologic cancers).
Targeted therapy: how revolutionary is it?
It is important to keep in mind that some older, empirically discovered agents are actually quite targeted-eg, camptothecin derivatives that target topoisomerase I.[5] On the other hand, some “targeted therapies” that were designed to be directed against a specific target have been shown to have clinical utility for unrelated reasons (eg, sorafenib [Nexavar] was not effective as a BRAF inhibitor; its utility likely stemmed instead from effects on the vascular endothelial growth factor [VEGF] receptor [VEGFR]). Finally, recent successes in cancer treatment may yet come from empirically derived chemical entities (eg, bendamustine [Treanda], which is active against a number of hematologic malignancies).
Renal Cell Carcinoma
1. What is the underlying tumor biology that is being targeted?
Early observations that patients with metastatic renal cell carcinoma (RCC) who underwent nephrectomy could experience spontaneous regression of pulmonary metastasis aroused interest in immune therapy in RCC. In fact, the only US Food and Drug Administration (FDA)-approved medications before targeted therapies were all immune therapies. However, the toxicities of such treatments were often formidable, and the responses were low and unpredictable.
Preclinical studies by Kaelin et al established a relationship between mutations in von Hippel-Lindau (VHL) genes and the failure of cells to degrade hypoxia-inducible factor-1α (HIF-1α).[6] This insight into RCC biology eventually established it as the poster child for heretofore largely resistant diseases in which a targeted therapy had demonstrated success: its median overall survival of 9 months in pre−targeted drug therapy trials[7-9] has been superseded by a median overall survival time of 22 months, with rapidly evolving diverse options for targeted therapies that are refining concepts of pathway inhibition.
FIGURE 1
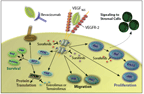
Angiogenic Pathways and Inhibitors Used in the Treatment of Renal Cell Carcinoma
In addition, RCC is a striking example of how knowledge of hereditary cancers has led to insight into pathways that are deranged in sporadic cancers. One common mutation in hereditary RCC (in VHL) results in an autosomal-dominant disease with a high lifetime likelihood of RCC.[10] VHL mutations also occur in up to three-quarters of sporadic RCC with clear-cell histology,[11] and accumulation of HIF-1α is a powerful activator of downstream signals involved in angiogenesis. These factors include not only VEGF, but also platelet-derived growth factor (PDGF) and the corresponding receptors (VEGFR and PDGFR). VEGF, PDGF, VEGFR, and PDGFR have all now become the therapeutic targets of tyrosine kinase inhibitors (TKIs). A second prominent pathway in RCC development was revealed in another autosomal-dominant inherited disease that frequently co-occurs with non−clear-cell RCC: tuberous sclerosis. Genetic mutations in tuberous sclerosis lead to inactivation of downstream Rheb protein, which in turn causes activation of mammalian target of rapamycin (mTOR) (Figure). mTOR plays a critical role in the development, progression, and metastatic potential of RCC, and it has also served as a successful target in the treatment of RCC, including the non−clear-cell subtype. mTOR exists in two forms: mTOR complex 1 (mTORC-1) and mTOR complex 2 (mTORC-2). mTORC-1 is the best understood form of mTOR, and it is downstream of the phosphatidylinositol-3'-kinase (PI3K)/AKT pathway. Its activation leads to activation of the p70 ribosomal protein S6 kinase (p70S6K) pathway, and activates angiogenesis through transcription of HIF-1α.[12,13]
2. How ‘targeted’ are the so-called ‘targeted drugs’?
TABLE 1
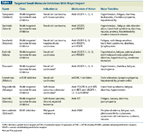
Targeted Small-Molecule Inhibitors With Major Impact
The first approved targeted therapy in RCC was sunitinib (Sutent), a multitargeted TKI that focuses mostly on the angiogenesis pathway, which encompasses VEGFR and PDGFR. In a 2005 clinical trial,[14] it demonstrated progression-free survival (PFS) and response advantages over the old standard therapy, interferon alfa (IFN-α), in treatment-naive patients with RCC. This was soon followed by the approval of a number of somewhat related agents that have different toxicity profiles and that may be active after the failure of other agents (Table 1). For example, sorafenib, a TKI that potently blocks VEGFR-2, VEGFR-3, PDGFR, and fibroblast growth factor receptor (FGFR), won approval for superior PFS, compared to placebo, in patients who had been exposed to cytokine therapies.[15] FGFR is a receptor that activates an alternate angiogenic pathway after the VEGF-related axis has been inhibited, and the fact that sorafenib targets FGFR may explain the activity of this agent in sunitinib failures. In addition, bevacizumab (Avastin; a monoclonal antibody against VEGF) and pazopanib (Votrient; a TKI that blocks all three isoforms of VEGFR [1,2, and 3]) both showed activity either in combination with IFN or as single agents in treatment-naive RCC.[16,17] In 2011, axitinib (Inlyta; a TKI targeting VEGFR-1, -2, -3, and PDGFR), another anti-angiogenic drug, showed longer PFS than sorafenib in the second-line setting.[18] In 2012, tivozanib, a TKI targeting VEGFR-1, -2, and -3, with potent activity and a long half-life, offered longer PFS than sorafenib as first-line treatment in patients with RCC,[19] again indicating the pivotal role of targeted therapy in combating angiogenesis in the treatment of RCC.
As the only first-line targeted therapy in RCC with an overall survival advantage over IFN-α, temsirolimus (Torisel), the first mTOR inhibitor to target mTORC-1, brought hope to patients with RCC, including to those with the non–clear-cell subtype.[20] Not surprisingly, everolimus (Afinitor), the first oral mTOR inhibitor that also targets mTORC-1, now is approved for patients who failed an anti-angiogentic drug, since it demonstrated a PFS advantage in the second-line setting.[21]
3. Is the targeted therapy also suitable for immunomodulation and/or immunoconjugation?
This progress based on TKIs and mTOR inhibitors should not blunt further research into immune modulation; IFN-α and interleukin-2 (IL-2) were the FDA-approved medications for RCC until the emergence of targeted therapy. The anti-angiogenic agent sunitinib has been shown to inhibit myeloid-derived stromal cells; this inhibition in turn reduces regulatory T-cell function, thus also providing immunomodulatory effects.[11] On the other hand, sorafenib inhibits the immunosuppression induced by myeloid-derived stromal cells, and can therefore potentially enhance immune function.[11] The mTOR inhibitor temsirolimus is derived from rapamycin analogs (“rapalogs”); it has been the chief component in immune modulation post organ transplantation, and it could have a significant role in immune modulation in RCC as well. Consequently, targeted therapies may also contribute to immune modulation, and their immunomodulatory effects can be utilized in the design of combination novel targeted therapies in RCC.
4. In what way does the targeted therapy constitute a meaningful improvement over chemotherapy?
Targeted therapy has changed metastatic RCC from a disease with nearly invariable and early fatality and with extremely limited yet very toxic treatment options (such as IL-2 and IFN) into a condition that is treatable for several months to years with a variety of therapeutic agents that offer reproducible efficacy to the majority of patients, with less toxicity. Before these agents were introduced, no prior chemotherapy trial had demonstrated even a prolongation in PFS, either alone or in combination, while this endpoint has been met by all FDA-approved targeted therapies, some of which have also demonstrated overall survival advantages. Of additional importance, these achievements have led to separate RCC treatment strategies based on histology and prognostic features, and to the application of different targeted therapies in sequence. This conceptual advance-coupling treatment to the biology of RCC-represents a key direction of future cancer therapy. RCC is also leading the way in the drive to establish a rational sequential use of these therapies on the basis of biological features; IFN has been successfully displaced, and only highly selected patients now receive interleukin-2 (IL-2).
Hepatocellular Carcinoma
1. What is the underlying tumor biology that is being targeted?
Systemic therapy of advanced hepatocellular carcinoma (HCC) had left behind a long record of negative phase III trials until the landmark placebo-controlled Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial demonstrated an impact of sorafenib on survival.[22] Advances in the imaging studies used in both the diagnosis and therapy of HCC had identified the development of the typical hypervascularity of HCC in the arterial phase, which is followed by rapid washout in the venous phase on a contrast-enhanced CT scan of the liver on a cirrhotic background. Such findings not only spurred the use of local measures against these lesions, but also gave rise to trials targeting tumor angiogenesis. The tumor itself carries frequent mutations in many tumor suppressor genes throughout its development and progression; these, coupled with the frequently compromised liver function (varying with the etiology of the liver disease), account for HCC’s notorious chemotherapy resistance. In fact, as the tumor progresses and acquires adverse prognostic features, such as vascular invasion and portal vein thrombosis, angiogenesis continues to play a critical role in metastasis and invasion. VEGF-A is one of the most potent angiogenetic factors in HCC; consequently, inhibition of VEGF-A is a key targeted therapy in HCC. In addition, basic fibroblast growth factor (bFGF) is also over expressed in HCC, and it may, along with VEGF-A, further enhance angiogenesis.[23,24] There are at least two other types of growth factor that play an important role in angiogenesis in HCC: PDGF, which stimulates formation of blood vessels, and angiopoietins, which modulate blood vessel vascularity to enhance nutrient delivery to tumor cells.[25] In addition to contributing to angiogenesis, epidermal growth factor receptor (EGFR) may also be important to the growth and metastasis of some HCCs: EGFR activation leads to activation of the downstream RAF/extracellular signal–regulated kinase (ERK) pathway and the PI3K/mTOR pathway. The mTOR pathway is a crucial part of hepatocyte malignant transformation into HCC.[26] Aberrant expression of mTOR has been demonstrated in up to 50% of HCC tissues,[27] and when mTOR becomes constitutively activated, it is associated with activation of the insulin-like growth factor (IGF) axis and upregulation of the PI3K pathway.[28] The mTOR pathway has become one of the therapeutic targets in HCC clinical trials. Raf kinase is also upregulated in HCC, representing another rational target for HCC treatment. Another possible therapeutic target-c-MET-the sole receptor of hepatocyte growth factor, is overexpressed in up to 49% of HCCs, and is associated with worse overall survival.[29]
2. How ‘targeted’ are the so-called ‘targeted drugs’?
In 2006, a new stage in HCC treatment unfolded when sorafenib improved overall survival in patients with unresectable HCC. Sorafenib is the first and only agent in the history of HCC therapy that has demonstrated an overall survival benefit; this targeted treatment-which inhibits VEGF-2, VEGF-3, PDGFR, and Raf kinases-has transformed the landscape of HCC treatment. Subsequent phase II studies using bevacizumab and erlotinib (Tarceva; a TKI targeting EGFR) in HCC have shown promise with regard to disease stabilization and PFS. Current studies of a PI3K inhibitor and mTOR inhibitors are ongoing, making treatment of HCC the prototype for targeted therapy.
3. Is the targeted therapy also suitable for immunomodulation and/or immunoconjugation?
Similar to RCC, HCC is a tumor with potential to respond to immunomodulation, and there are case reports of spontaneous regression. Immune therapy has figured prominently in past HCC treatments: IFN was tested as a component of combination therapy for HCC (IFN, cisplatin, doxorubicin, and fluorouracil [5-FU]), but high response rates did not confer an overall survival benefit. New studies are focusing on glypican-3, a member of the proteoglycan family that is found on the surface of HCC cells, and which is not present in nonmalignant tissue.[30] A phase I study in patients with advanced HCC used a peptide vaccine against glypican-3; the study demonstrated that patients with a cytotoxic T-cell lymphocyte frequency (percentage of both glypican-3 peptide+ and CD8+ T cells pre- and post-vaccination) of ≥ 50 had longer overall survival than those whose cytotoxic T-cell lymphocyte frequency did not reach 50.[31] There is potential interest in immunotherapy for the treatment of HCC because of the possibly lesser risk of incurring toxicities in the setting of precarious liver dysfunction.
4. In what way does the targeted therapy constitute a meaningful improvement over chemotherapy?
The key feature of targeted therapy in HCC has been the demonstration that it can offer clear survival advantages to patients undergoing treatment. With sorafenib, for the first time, patients with limited options because of known liver disease have been able to experience clinical benefit. This experience highlights the inadequacies of response rate as a reflection of benefit for patients with this disease. In fact, lack of objective tumor regression should not diminish our confidence in the efficacy of targeted therapy. By contrast, while chemotherapy drug regimens could claim higher response rates, they all failed to prolong overall survival in HCC patients.
Malignant Melanoma
1. What is the underlying tumor biology that is being targeted?
Other than the known propensity of melanoma to accelerate its growth years after a latent stage, virtually little information of much therapeutic use had been assembled in at least three decades. Two breakthroughs initiated a cascade of events leading to successful clinical applications that were covered by both The New York Times and the American Society for Clinical Oncology (ASCO) in 2010.
One powerful lead was related to a BRAF mutation. BRAF, a member of the MAP kinase signaling pathway, is involved in cell proliferation, differentiation, and apoptosis. Up to 60% of melanomas have a V600 mutation in BRAF, leaving the kinase in a constitutively active form.[32] Identification of this pathway as one that confers a growth advantage, leading to accelerated growth in the presence of additional mutations, was demonstrated in mouse models [33].
TABLE 2

Targeted Monoclonal Antibodies With Major Impact
Another approach-trying to harness immunity against tumors-led to investigation of the importance of the cytotoxic T lymphocyte antigen-4 (CTLA-4) receptor on T cells. Antibodies to CTLA-4 prevent the negative regulation of the T-cell interactions with antigen-presenting cells. Antigen-presenting cells use CD-28 to help bind T cells while presenting peptide fregments to T cells during this positive interaction. CTLA-4 expression then becomes upregulated on the T-cell surface, and CTLA-4 binds B7 on tumor cells, which bears the antigen more tightly than CD-28 (Table 2), thereby effectively slowing the activated immune system, acting in a manner similar to that of a brake. The immunologic consequence of removing the “brake” that CTLA-4 puts on the immune system is the unleashing of T-cell–mediated immunity; this stimulation was further shown to be clinically relevant. The intense interest emanating from the unprecedented clinical successes that followed these discoveries has resulted in further inquiries into the tumor biology of melanoma and in additional developments, such as the simultaneous use of B-raf and MEK inhibitors.[34]
2. How ‘targeted’ are the so-called ‘targeted drugs’?
Until the introduction of targeted therapy with ipilimumab (Yervoy; an antibody against CTLA-4) and vemurafenib (Zelboraf, a B-raf inhibitor), the treatment of malignant melanoma in the adjuvant setting was limited to IFN-and in the metastatic setting to high-dose IL-2, radiation, and cytotoxic chemotherapy. All of these therapies, even though some were “targeted,” had minimal impact on overall survival.
Vemurafenib is an extremely potent and specific inhibitor of the BRAF V600E activating mutation, providing an effective blockade of the downstream MAP kinase pathway. This compound, introduced by Plexxikon, as well as the similarly targeted drug by GlaxoSmithKline (dabrafenib), represent a remarkably successful feat in drug development. Compared to dacarbazine, vemurafenib increased 6-month overall survival: 84% of vemurafenib-treated patients survived compared with 64% of patients treated with dacarbazine.[35] Of interest, future directions of therapy include other methods of blockading the MAP kinase pathway, such as with new inhibitors of MEK.[36] Studies are underway to investigate whether dual blockade of both B-raf and MEK can overcome acquired resistance to B-raf inhibition.
Ipilimumab does not directly target melanoma; instead, by targeting CTLA-4, it prevents application of the “brake” and allows the body’s immune system to sustain an attack on malignant cells. At the same time, it allows the immune system to attack a number of other tissues-a cause of toxic manifestations (and paradoxically an indication of “non-specific targeting”). Compared with administration of a gp100 vaccine alone, ipilimumab increased overall survival in metastatic melanoma by close to 5 months.[37] While this effect is not specifically limited to melanoma, malignant melanoma is the first tumor in which deregulating the immune system has proven effective at curbing malignant cell growth. In a similar manner, more recent data have shown that curbing the negative regulation of T cells by inhibiting PD-1 or the PD-1 ligand (PD-L1) may also improve the cytotoxic attack of T cells on a number of malignant cells, including melanoma cells.[38,39]
3. Is the targeted therapy also suitable for immunomodulation and/or immunoconjugation?
Immunomodulation has a place in the treatment of malignant melanoma, and has been proven effective with the advent of CTLA-1 and PD-1 inhibition. Ipilimumab is likely to be a prototype for the harnessing of the immune system to target malignancies. Allowing T cells to act without functional inhibition leads to improvements in overall survival in patients with melanoma.
4. In what way does the targeted therapy constitute a meaningful improvement over chemotherapy?
For a decade, cytotoxic chemotherapy had minimal benefit in the treatment of melanoma. Even the combination of chemotherapy and nonspecific immunotherapy proved futile. BRAF and MEK inhibition have shown their impressive power in halting disease progression. Vemurafenib has been proven to be more effective in the treatment of this disease than the standard cytotoxic agent, dacarbazine. Furthermore, with research in melanoma focusing squarely on targeting the MAP kinase pathway and harnessing the immune system to combat the disease, it is likely that we have seen the end of cytotoxic chemotherapy use for melanoma. In malignant melanoma, targeted therapy is the only meaningful treatment option currently available.
GIST and Other Sarcomas
We include sarcomas as a “major impact area” because even the notoriously chemo-resistant tumors that are prevalent in adults are increasingly being reclassified based on molecular profiling, leading to totally new therapeutic paradigms. GIST was the initial major success of targeted therapy-once a potent inhibitor of an activated tyrosine kinase such as imatinib was identified in laboratory studies[40] and tried in a patient with advanced GIST while the drug was being tested as an inhibitor of BCR/ABL in chronic myeloid leukemia. GIST became the prototype for a notoriously chemo-resistant sarcoma that yielded to a targeted agent; the approach that led to imatinib’s success in GIST was subsequently extended to other c-kit–activating mutations. Sunitinib, sorafenib, and more recently regorafenib[41] have shown efficacy in GIST imatinib failures-regorafenib presumably by successfully targeting PDGFR-A as well as KIT, which are implicated in GIST growth and imatinib resistance.[42] In addition, other tumors of mesenchymal origin, such as dermatofibrosarcoma protuberans, have been shown to be responsive to imatinib because their growth is in part driven by PDGFR.[43] A number of sarcoma subtypes have been defined by chromosomal translocations coding for chimeric oncoproteins that promote abnormal transcription; typical translocations have been described in synovial sarcoma, epithelioid sarcoma, and Ewing sarcoma. Ewing sarcoma is associated with the rearrangement of the EWS gene, which results in IGF-1 or IGF-2 hyperactivation and susceptibility to inhibitors of the IGF pathway.[44] Alveolar soft part sarcoma’s translocation results in a fusion protein that is associated with MET autophosphorylation and activation of downstream pathways, which promote growth and angiogenesis; it has been successfully targeted by sunitinib, as well as other drugs that inhibit VEGFR-1.[45,46] The growth of giant-cell tumors is mediated by RANK-ligand (RANKL)-induced osteoclast activation; denosumab (Xgeva), an inhibitor of RANKL, is able to reverse this tumor’s locally aggressive behavior.[47] The perivascular epithelioid cell tumors (PEComas) and related lymphangioleiomyomatosis occur as part of tuberous sclerosis complex (TSC), in which mutations in TSC1 or TSC2 result in defective negative regulation of mTOR; treatment with rapalogs has been effective.[48] Trabectedin (Yondelis) is a marine product that inhibits transcription-coupled DNA repair, leading to responses in myxoid liposarcoma,[49] a tumor with a unique chromosomal rearrangement that also confers increased sensitivity to ifosfamide and doxorubicin.[50] Pazopanib has recently been approved as a systemic treatment for the notoriously drug-resistant leiomyosarcomas.[51] Finally, paclitaxel-as well as anti-angiogenic strategies-although disappointing in many forms of sarcoma,[52] has activity against angiosarcomas.[53]
Considered in perspective, the systemic treatment of sarcomas had changed little since the landmark introductions of doxorubicin and ifosfamide for soft-tissue sarcomas and cisplatin for bone sarcomas. It is now mandatory to consider specific therapeutic targets when a tumor of mesenchymal origin requiring systemic therapy is first identified.
Financial Disclosure:Dr. Muggia has served on safety data monitoring committees for Bayer, Lilly, Pfizer, and Roche. Dr. Wu and Dr. Joseph have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentined in this article.
References:
REFERENCES
1. DeVita VT, Jr. Rosenberg SA. Two hundred years of cancer research. N Engl J Med. 2012;366:2207-14.
2. Tuma RS. Strides in melanoma announced: maximizing value comes next. J Natl Cancer Inst. 2011;103:997-9.
3. Schilsky RL. Personalizing cancer care: American Society of Clinical Oncology presidential address 2009. J Clin Oncol. 2009;27:3725-30.
4. Schilsky RL. Personalized medicine in oncology: the future is now. Nat Rev Drug Discov. 2010;9:363-6.
5. O'Leary J, Muggia FM. Camptothecins: a review of their development and schedules of administration. Eur J Cancer. 1998;34:1500-8.
6. Kaelin WG, Jr. The von Hippel-Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res. 2007;13:680s-84s.
7. George CM, Vogelzang NJ, Rini BI, et al. A phase II trial of weekly intravenous gemcitabine and cisplatin with continuous infusion fluorouracil in patients with metastatic renal cell carcinoma. Ann Oncol. 2002;
13:116-20.
8. Porta C, Zimatore M, Imarisio I, et al. Gemcitabine and oxaliplatin in the treatment of patients with immunotherapy-resistant advanced renal cell carcinoma: final results of a single-institution phase II study. Cancer. 2004;100:2132-8.
9. Van Veldhuizen PJ, Faulkner JR, Lara PN, Jr, et al. A phase II study of flavopiridol in patients with advanced renal cell carcinoma: results of Southwest Oncology Group Trial 0109. Cancer Chemother Pharmacol. 2005;56:39-45.
10. Maher ER. Von Hippel-Lindau disease. Curr Mol Med. 2004;4:833-42.
11. Milella M, Felici A. Biology of metastatic renal cell carcinoma. J Cancer. 2011;2:369-73.
12. Coppin C. Everolimus: the first approved product for patients with advanced renal cell cancer after sunitinib and/or sorafenib. Biologics. 2010;4:91-101.
13. Lane HA, Wood JM, McSheehy PM, et al. mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res. 2009;15:1612-22.
14. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115-24.
15. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125-34.
16. Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-8.
17. Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28:2137-43.
18. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931-9.
19. Motzer RJ, Nosov D, Eisen T, et al; and the TIVO-1 Study Group. Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial. J Clin Oncol. 2012;30(Suppl):abstr 4501.
20. Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271-81.
21. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-56.
22. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-90.
23. Yoshiji H, Kuriyama S, Yoshii J, et al. Synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma. Hepatology. 2002;35:834-42.
24. Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007;72(Suppl 1):30-44.
25. Tanaka S, Mori M, Sakamoto Y, et al. Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J Clin Invest. 1999;103:341-5.
26. Villafuerte BC, Goldstein S, Robertson DG, et al. Nutrition and somatomedin XXIX. Molecular regulation of IGFBP-1 in hepatocyte primary culture. Diabetes. 1992;41:835-42.
27. Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972-83, 83 e1-11.
28. Wu J, Zhu AX. Targeting insulin-like growth factor axis in hepatocellular carcinoma. J Hematol Oncol. 2011;4:30.
29. Ueki T, Fujimoto J, Suzuki T, et al. Expression of hepatocyte growth factor and its receptor, the c-met proto-oncogene, in hepatocellular carcinoma. Hepatology. 1997;25:619-23.
30. Anatelli F, Chuang ST, Yang XJ Wang HL. Value of glypican 3 immunostaining in the diagnosis of hepatocellular carcinoma on needle biopsy. Am J Clin Pathol. 2008;130:219-23.
31. Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686-96.
32. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-19.
33. Dankort D, Filenova E, Collado M, et al. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379-84.
34. Sausville EA. Promises from trametinib in RAF active tumors. N Engl J Med. 2012;367:171-2.
35. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;
364:2507-16.
36. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-14.
37. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
38. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-54.
39. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-65.
40. Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408-17.
41. George S, Wang Q, Heinrich MC, et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol. 2012;30:2401-7.
42. Bosbach B, Deshpande S, Rossi F, et al. Imatinib resistance and microcytic erythrocytosis in a KitV558{Delta};T669I/+ gatekeeper-mutant mouse model of gastrointestinal stromal tumor. Proc Natl Acad Sci U S A. 2012;109:E2276-83.
43. Komdeur R, Hoekstra HJ, Molenaar WM, et al. Clinicopathologic assessment of postradiation sarcomas: KIT as a potential treatment target. Clin Cancer Res. 2003;9:2926-32.
44. Arnaldez FI, Helman LJ. Targeting the insulin growth factor receptor 1. Hematol Oncol Clin North Am. 2012;26:527-42, vii-viii.
45. Vistica DT, Hollingshead M, Borgel SD, et al. Therapeutic vulnerability of an in vivo model of alveolar soft part sarcoma (ASPS) to antiangiogenic therapy. J Pediatr Hematol Oncol. 2009;31:561-70.
46. Stacchiotti S, Negri T, Zaffaroni N, et al. Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol. 2011;22:1682-90.
47. Branstetter DG, Nelson SD, Manivel JC, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012;18:4415-24.
48. Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835-40.
49. Cesne AL, Cresta S, Maki RG, et al. A retrospective analysis of antitumour activity with trabectedin in translocation-related sarcomas. Eur J Cancer. 2012; Jun 28 [Epub ahead of print].
50. Katz D, Boonsirikamchai P, Choi H, et al. Efficacy of first-line doxorubicin and ifosfamide in myxoid liposarcoma. Clin Sarcoma Res. 2012;2:2.
51. Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. 2009;27:3126-32.
52. Casper ES, Waltzman RJ, Schwartz GK, et al. Phase II trial of paclitaxel in patients with soft-tissue sarcoma. Cancer Invest. 1998;16:442-6.
53. Park MS, Ravi V, Araujo DM. Inhibiting the VEGF-VEGFR pathway in angiosarcoma, epithelioid hemangioendothelioma, and hemangiopericytoma/solitary fibrous tumor. Curr Opin Oncol. 2010;22:351-5.