Thymoma: Current Concepts
This review considers the status of the different histological classifications thus far presented for thymomas and offers an analysis of the association between histology and clinical behavior.
Thymomas are unusual tumors, representing no more than 1% of all malignancies. However, thymomas are the most common epithelial tumors of the anterior mediastinum. Unfortunately, there is no general agreement regarding the best parameters to use to predict clinical behavior in these tumors. This review considers the status of the different histological classifications thus far presented for thymomas and offers an analysis of the association between histology and clinical behavior. It also emphasizes the importance of proper staging of thymomas, delineating the benefits and shortcomings of different proposed staging systems and offering thoughts on a better and more accurate staging stratification for patients with these tumors. All of the different parameters are presented in relation to survival rates. Based on current information, staging with proper stratification remains the most important parameter for predicting prognosis. For tumors limited to the mediastinal compartment, surgical resection is the most effective treatment, while induction therapy is a good alternative for patients in whom surgical resection is not possible.
Introduction
Thymomas are among the most common tumors of the anterior mediastinum. However, considered in the context of the entire spectrum of malignancies, thymomas are unusual neoplasms. It is important to note that thymoma does not represent the benign counterpart of thymic carcinoma, nor is thymic carcinoma the malignant counterpart of thymoma. These two neoplasms, thymoma and thymic carcinoma, represent two different clinicopathological entities, which have been shown to be associated with different clinical outcomes. It is also important to point out that these two conditions may be treated differently depending on the clinical circumstances at the time of diagnosis.
Over the last two decades, controversy has arisen over several views that have been presented on the histological classification, staging, treatment, and clinical behavior of thymomas. As more knowledge is gathered from the different specialties involved in the treatment of patients with thymomas, some views on this subject are likely to be upheld while others will not be, with the result that patients with this neoplasm will receive better care. However, it is important to note that due to the rarity of thymomas, a concentrated experience with a large number of cases is difficult to assemble.
Clinical Aspects
Although the symptomatology that patients with thymoma exhibit on presentation is varied, it is worthwhile highlighting the association of thymomas with paraneoplastic syndromes. Perhaps the most important of these syndromes, or at least one that appears to be among those most frequently associated with thymoma, is myasthenia gravis. Approximately 10% to 15% of patients with myasthenia gravis have thymoma; conversely, about 40% of patients with thymoma have an associated paraneoplastic syndrome, of which myasthenia gravis is among the most common. Other paraneoplastic syndromes seen in patients with thymoma include limbic encephalitis, neuromyotonia, stiff person syndrome, myotonic dystrophy, and polymyositis.[1] Thymoma patients may also have a wide spectrum of associated non-neurologic conditions, including myocarditis, graft-versus-host disease, hypogammaglobulinemia, pure red cell aplasia, intestinal pseudo-obstruction, mixed connective tissue disease, thyroiditis, and many more.[1]The prognosis of patients with thymoma is closely linked to the underlying condition.
Patients affected with thymoma may also present with signs and symptoms unrelated to any underlying condition, including such nonspecific symptoms as chest pain, cough, dyspnea, and other symptoms of mediastinal compression.When symptoms such as these are seen, the initial study to use in evaluation of the patient is chest radiography. Chest imaging will disclose the presence of an anterior mediastinal mass, which can be further analyzed using more advanced studies, such as chest CT, a procedure that provides more specific information regarding the circumscription or invasive nature of the neoplasm.[2]
Histological Classifications
TABLE 1

Different Histological Classifications of Thymomas and the WHO Schema
Several histological approaches have been proposed for the classification of thymomas. Some of the most popular are shown in Table 1. The basic purpose of these schemas essentially has been to provide information that may be used as a parameter for predicting clinical behavior. However, while some of these classification schemas have become popular, they have not gained universal acceptance.
In 1961, Bernatz et al, of the Mayo Clinic,[3] proposed a histological classification of thymomas that has become known as the “traditional” classification. The authors separated thymomas based on the amount of lymphocytic component. Thus, a tumor in which the lymphocytic component is predominant is classified as a lymphocyte-rich thymoma; a tumor in which the lymphocytic component and the epithelial component are in about the same proportion is classified as a mixed thymoma or a lymphoepithelial thymoma; and a tumor in which the lymphocytic component is scant, with a predominance of epithelial cells, is classified as an epithelial-rich thymoma. Because all of these subtypes are characterized by epithelial cells that are round to oval, the authors also added another thymoma to their classification, a tumor they designated as “spindle-cell thymoma” because the shape of the cells appears more elongated (fusiform). Interestingly, even though this schema allows for easy classification of the different types of thymomas, Bernatz et al also stated that all of the subtypes included in the schema have the potential to become invasive tumors, thereby arguing in favor of staging as the best predictor of prognosis regardless of a tumor’s histological subtype.[4]
FIGURE 1
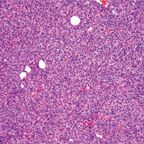
Spindle-Cell ThymomaFIGURE 2
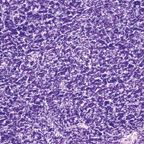
ThymomaFIGURE 3
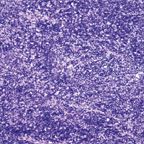
ThymomaFIGURE 4
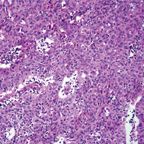
Atypical Thymoma
In 1983, Marino and Mller-Hermelink[5] presented a new approach to the classification of thymomas, separating them into cortical, medullary, and mixed thymomas. This classification is also known as the “histogenetic classification,” since the authors purportedly considered that the thymic epithelial cells of the medulla and those of the cortex are the ones that specifically give rise to the different types of thymomas they describe. However, this assumption has not been verified by the authors of the classification or by anyone else. Moreover, the histogenetic classification was constructed based on biopsy specimens rather than resected tumors, and it did not consider the heterogeneity of thymomas, a fact that not only is well known but also has been amply documented in the literature.[6,7] Even more important to point out with regard to the histogenetic classification is the fact that the authors believe that certain histologies correlate with and thus predict certain outcomes, regardless of the staging of the tumor at the time of diagnosis.[5,8] This approach clearly differs from approaches in which tumor staging is regarded as a predictor of outcomes.
Because of the differing views on the classification of thymomas, a panel of experts was convened by the World Health Organization (WHO) in 1999[9] to shed some light on the nature of these tumors, with the hope of providing a unifying approach to their classification. What the WHO panel produced was an uncommitted schema that used letters and numbers, and that could be used as a “translator” of the only two classification systems that were discussed-the Bernatz and the Mller-Hermelink. Type A thymoma replaced the spindle-cell or medullary thymoma, while type B thymomas were given number designations from 1 to 3 depending on the size of the lymphocytic component (Figures 1-4). These letters and numbers, as clearly stated in the publication, did not represent a new classification but merely a “translator” for the different classifications already in existence. Unfortunately, other important issues, such as sampling, were never discussed or addressed by this group. Interestingly, in the new version of the WHO classification, which was published in 2004,[10] the schema of letters and numbers was unofficially introduced as “the classification of thymomas”; still more interesting in this publication is the attaching of a degree of aggressiveness to certain histological groups, once again favoring histology over staging.
Also in 1999, Suster and Moran[11] presented a novel conceptual approach to the classification of thymic epithelial neoplasms, separating these tumors into three main categories: thymoma, atypical thymoma, and thymic carcinoma. In addition, in opposition to the idea of histology as a predictor of clinical behavior, they stressed that staging is a more reliable parameter for predicting outcomes. The authors did note that some tumors, namely the atypical thymomas, may have a greater tendency to become invasive and more aggressive than do the other types of thymomas; consequently, they emphasized the need to separate out this particular subtype. However, they maintained that staging nonetheless remains the single most important parameter for predicting outcome.
FIGURE 5
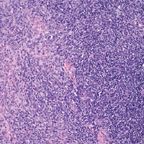
Thymoma Displaying Mixed Histologies
Even though it appears that these classification systems by themselves represent only a philosophical approach to the classification of these tumors, in fact, they go beyond a simple stratification of thymomas. For instance, in the Mller-Hermelink classification and in the WHO schema, the spindle-cell thymoma of the Bernatz classification (labeled as “medullary thymoma” or “WHO type A,” respectively, in the former schemas) is considered to be a benign neoplasm. This is not only misleading but in fact inaccurate. Recently, Moran et al,[12] using very stringent criteria for tumor classification, reported a series of 41 cases of spindle-cell thymoma (medullary thymoma), all of which were invasive neoplasms in different stages of invasion, thus clearly demonstrating that these tumors can be just as aggressive as the other subtypes of thymomas. The authors draw attention to the fact that staging still is the best predictor of prognosis.
FIGURE 6
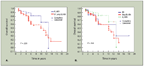
A) Comparison of Overall Survival by Histologic Subtype-for Types A and AB1 vs Types B1, B2, and B3
One other issue that recently has been convincingly demonstrated is the heterogeneity of these tumors. In all previous publications in which a system for the classification of thymomas has been set forth, including the WHO schema, the issue of tumor sampling has been ignored. In a recent series of 250 thymoma cases,[13] it has been demonstrated, using specific tumor sampling criteria (at least 5 sections of tumor for classification), that more than 50% of thymomas display two or more different growth patterns and cannot be placed into a single slot such as those proposed by the WHO (Figure 5). These thymomas will invariably be diagnosed as mixed histology thymomas, clearly arguing against the notion that histology is a predictor of clinical behavior. Interestingly, when statistical analysis was performed in the cases that demonstrated mixed histology in an attempt to determine whether there was any statistical correlation between histology and clinical behavior, none was found (Figures 6A, 6B).
Currently, based on these findings, staging of the tumor at the time of diagnosis not only appears to be the single most important predictor of clinical outcome but also one that can be used for proper stratifications of patients who may need additional therapy beyond surgical resection of the tumor.
Staging Systems
TABLE 2

Different Histological Classifications of Thymomas and the WHO Schema
Just as with histological classifications, different staging systems have been proposed with the goal not only of predicting clinical behavior but also facilitating the proper stratification of patients who may need additional medical treatment.[14-19] Even though most of these staging systems essentially deal with the circumscription or invasiveness of the tumor at the time of diagnosis, for the last few decades the staging system proposed by Masaoka in 1981 has been the most popular.[18] However, from the pathologist’s perspective, the Masaoka approach is not the easiest system to use; it relies heavily on macroscopic findings that may be apparent to a surgeon in the operating room but are less so to the pathologist who eventually will have to determine whether the tumor is encapsulated or not. In view of the shortcomings of such an approach, a group of pathologists and thoracic surgeons recently joined together to propose a pathological system based on a collected experience of 250 thymoma cases.[20] This staging system not only is reproducible by pathologists, surgeons, and other clinicians, but in addition, it provides crucial information for the stratification of patients who may benefit from additional medical treatment. Also important is the fact that in this new proposed staging system, the encapsulated thymoma is categorized as stage 0, emphasizing the premalignant nature of the neoplasm. In addition, the most important feature of this proposed new staging system is the separation of limited and invasive disease. Furthermore, in the invasive disease category, the clear separation of different anatomic structures not only should be followed with ease by pathologists and clinicians but, again, also will allow for better stratification of patients who may benefit from additional therapy. Table 2 compares the Masaoka and Moran approaches to the staging of thymomas.
Treatment
There is general agreement that the treatment of thymomas is primarily surgical. However, that approach is reserved for tumors that are either encapsulated or limited to the mediastinal region, so that complete surgical resection can be performed. Given this goal for surgical treatment, surgeons need to rely not only on a previous biopsy but also on radiological imaging, with both pointing to a diagnosis of thymoma confined to the anterior mediastinum. If that is the case, then complete surgical resection is the proper treatment option.
Problems arise when tumors are deemed to be invasive or just outright unresectable. For patients in whom the tumor is determined to be invasive, there are several possibilities that should be mentioned. Radiation therapy has been used either as a postoperative treatment to diminish the risk of recurrence, as treatment for patients who cannot undergo surgical resection, or as an adjuvant treatment for patients who have undergone induction chemotherapy. Nevertheless, the use of radiation therapy for invasive tumors remains controversial, as there are conflicting views regarding its benefits.[15,21,22] There is evidence of long-term risk of cardiovascular disease in patients who have received mediastinal radiation. Induction therapy may include radiation alone, combined radiation and chemotherapy, or chemotherapy alone. The use of neoadjuvant chemotherapy alone has been tried with differing degrees of success in patients with invasive tumors who are otherwise good candidates for surgical resection. Several series of invasive thymomas treated with regimens including cyclophosphamide, doxorubicin, cisplatin, and prednisone have been reported, with responses varying from 75% to 100%. In these series of cases, the number of patients in each individual study varied between 3 and 22, and in all patients, treatment was followed by surgical resection of the tumor.[23-32] Two of the largest series of cases reporting experience with this modality are the studies by Rea et al and Kim et al.[23,32] Rea et al[32] reported on 16 patients with invasive thymoma in Masaoka stages III or IVA who were treated with chemotherapy followed by surgical resection. The chemotherapy was administered in 4-day courses and the courses were repeated every 3 weeks. The authors reported complete remission in 7 patients (43%) and partial remission in 9 patients (57%). In addition, the authors documented a median survival of 66 months, with a 3-year survival of 70%. Kim et al[23] reported on 22 patients with invasive thymomas in Masaoka stages III, IVA, or IVB who were treated with induction chemotherapy, surgical resection, radiation therapy, and three courses of consolidation chemotherapy. The authors documented that 18 of 19 patients who completed the multidisciplinary approach were disease-free after 50.3 months. In addition, the authors documented overall survival rates of 95% at 5 years and 79% at 7 years, with progression-free survival rates of 77% at 5 and 7 years. Based on these results, the authors concluded that the use of induction chemotherapy to optimize surgical resection, followed by radiation therapy and consolidation chemotherapy, leads to good control of residual disease and high overall survival rates.
However, more recently, Wright et al[26] reported on a retrospective analysis of 10 patients who had received neoadjuvant chemotherapy before surgical resection. The documented response, based on tumors with 90% necrosis or more, was that 40% (4 out of 10 patients) had an estimated 5-year survival rate of 69%. With this in mind, it is very important to highlight a few issues concerning what is being regarded as a complete, or 100%, response. Essentially, response is determined by the amount of necrosis in a particular tumor. Thus, if a tumor is completely necrotic, then that is considered a complete response. Even though such an assessment has been made in some of the series reported thus far, it is significant that the pathological documentation, with regard to the extent of sampling, has been lacking. If a patient undergoes neoadjuvant chemotherapy for an invasive thymoma, it is believed that the tumor has invaded adjacent structures-and therefore is most likely a large tumor. Thus, to document 100% necrosis becomes an issue of sampling, and so far, there is not a gold standard as to how extensive the sampling should be in patients who have undergone neoadjuvant chemotherapy. In our experience with similar cases (28 cases-unpublished data), the amount of necrosis present has varied from 0 to about 90%, and if sampling has been sufficiently extensive, then invariably one is able to find residual areas of viable tumor. In addition, it appears that some subtypes of thymomas are more likely to undergo necrosis. For instance, thymomas with a lymphocytic component are more likely to show necrosis, in contrast to thymomas that lack the lymphocytic component, such as the spindle-cell thymoma or the atypical thymoma (WHO types A and B3, respectively). Therefore, it is possible to argue that if the histology of an invasive thymoma is one of those with a lymphocytic component, it is more likely to produce some sort of response. This particular point requires further analysis, as it may impact decisions as to who stands to benefit the most from neoadjuvant chemotherapy. One final factor warranting consideration is that some patients with thymomas may have tumors that could have undergone necrotic, cystic, and hemorrhagic changes without induction therapy,[33] thus adding to the difficulty of clinical assessment by necessitating the separation of de novo changes from those resulting from induction therapy.
Survival
Some individuals and organizations, such as the World Health Organization, have ascribed certain clinical behaviors to some tumors. For instance, the spindle-cell thymoma (WHO type A) is one of those tumors that is believed by some to be a “benign” tumor, while other tumors that show different proportions of lymphocytic component have been associated with varying degrees of aggressiveness. Unfortunately, a few issues have been misunderstood regarding thymomas. First, statistically speaking, the subtypes of thymomas that demonstrate a lymphocytic component are more common than those demonstrating a lack of lymphocytes.[13] Therefore, it is more likely that one would see tumors with a lymphocytic component behaving more aggressively. However, that does not mean that other tumors that are less common will behave less aggressively. On the contrary, atypical thymomas are thought to be more likely invasive tumors and more likely to recur. The nature of spindle-cell thymomas was recently clarified after the presentation of a series of 41 cases that were in different stages of invasiveness. In addition, it is worth underscoring the fact that more than 50% of thymomas will show mixed histologies if properly sampled, thus casting some doubt on the real significance of histological subtyping of thymomas in relation to prognosis. In a recent series of 250 thymoma cases, the issue of histology and staging was analyzed by statistical means.[13] When the relationship of the different histologies to survival was analyzed in these patients, the results obtained were not statistically significant. However, when the relationship of staging to survival was analyzed in the same patients, there were statistically significant results. More important, when such results were further analyzed, by classifying tumors (using Moran’s staging system) into limited disease vs invasive disease, the correlation with survival was even better. In general, one can consider that in patients with an encapsulated thymoma who undergo complete surgical resection, the prognosis and overall survival should be excellent and the risk of recurrence very minimal. In addition, with invasive tumors (Moran’s Stage I) that have breeched the capsule (ie, the capsular integrity has been compromised)-so-called minimally invasive thymomas-outcomes should be similar to those seen with encapsulated thymomas. In this setting the recurrence rate is below 10%. On the other hand, with invasive tumors not confined to the thymus, the recurrence rate is higher by as much as 40% (Figures 7A, 7B).
FIGURE 7

Prognosis Analyzed by Proposed Stages 0/1 vs II/III. A) Overall Survival Curve Showing a Statistically Significant Difference
In general terms, based on the collective experience represented by different reports on thymomas and their clinical behavior, it has been estimated that in thymomas confined to the mediastinum (Masaoka stage I and some stage II), the 10-year-and-greater survival rate averages slightly more than 80%.[20] Therefore, it is crucial that staging be properly determined at the time of diagnosis, since it is associated with patient survival. One other feature that has been evaluated as a possible predictor of clinical behavior has been the size of the tumor; however, no statistically significant results have been obtained using that specific parameter.
Conclusions
Even though issues involving thymomas are likely to continue to be discussed, based on current available information, one can conclude the following:
• Thymomas represent a different clinicopathological entity from thymic carcinoma.
• Histology alone does not correlate with better or worse prognosis in thymoma patients.
• Thymomas are heterogeneous tumors, likely to show mixed histologies in over 50% of cases, thus further limiting the use of histology alone as a predictor of clinical behavior.
• Pathologic staging appears to be the single most important prognostic factor in the evaluation of thymomas.
• Stratification of patients with invasive tumors into specific categories on the basis of the anatomic area compromised (following Moran’s staging system) may enable clinicians to be more selective regarding whom they offer additional treatment.
• It is very important that thymomas be sampled properly in order to avoid the possibility that a thymic tumor exhibiting both components-thymoma and thymic carcinoma-might be missed.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. Tormoehlen LM, Pascuzzi RM. Thymoma, myasthenia gravis, and other paraneoplastic syndromes. Hematol Oncol Clin N Am. 2008;22:509-26.
2. Rosado-de-Christenson ML, Strollo DC, Marom EM. Imaging of thymic epithelial neoplasms. Hematol Oncol Clin N Am. 2008;22:409-31.
3. Bernatz PE, Harrison EG, Claggett OT. Thymoma. A clinicopathologic study. J Thorac Cardiovasc Surg. 1961;42:424-44.
4. Bernatz PE, Khonsari S, Harrison EG, et al. Thymomas: factors influencing prognosis. Surg Clin North Am. 1973;53:885-92.
5. Marino M, Müller-Hermelink HK. Thymoma and thymic carcinoma: relation of thymoma epithelial cells to the cortical and medullary differentiation of thymus. Virchows Arch A Pathol Anat Histopathol. 1985;
407:119-49.
6. Salyer WR, Eggleston JC. Thymoma: a clinical and pathological study of 65 cases. Cancer. 1976;37:229-49.
7. Moran CA, Suster S. On the histologic heterogeneity of thymic epithelial neoplasms: impact of sampling in subtyping and classification of thymoma. Am J Clin Pathol. 2000;114:760-6.
8. Quintanilla-Martinez L, Wilkins EW, Choi N, et al. Thymoma: histologic subclassification is an independent prognostic factor. Cancer. 1994;74:606-17.
9. Rosai J. Histological typing of tumours of the thymus. 2nd ed. Berlin, Germany: Springer; 1999.
10. Travis WD, Brambilla E, Müller-Hermelink HK, et al eds. WHO classification of tumours: pathology and genetics of tumours of the lung, pleura, thymus, and heart. Lyon, France: IARC Press; 2004.
11. Suster S, Moran CA. Thymoma, atypical thymoma, and thymic carcinoma: a novel conceptual approach to the classification of thymic epithelial neoplasms. Am J Clin Pathol. 1999;111:826-33.
12. Moran CA, Kalhor N, Suster S. Invasive spindle cell thymomas (WHO type A): a clinicopathologic correlation of 41 cases. Am J Clin Pathol. 2010;134:793-8.
13. Moran CA, Weissferdt A, Kalhor N, et al. Thymoma I. A clinicopathologic correlation of 250 cases with emphasis on the World Health Organization Schema. Am J Clin Pathol. 2012;137:444-50.
14. Bergh NP, Gatzinsky P, Larsson S, et al. Tumors of the thymus and thymic region, I: clinicopathological studies on thymomas. Ann Thorac Surg. 1978;25:91-8.
15. Curran WJ, Kornstein MJ, Brooks JJ, et al. Invasive thymoma: the role of mediastinal irradiation following complete or incomplete surgical resection. J Clin Oncol. 1988;6:1722-7.
16. Gamondes JP, Balawi A, Greenland T, et al. Seventeen years of surgical treatment of thymoma: factors influencing survival. Eur J Cardiothorac Surg. 1991;5:124-31.
17. Yamakawa Y, Masaoka A, Hashimoto T, et al. A tentative tumor-node-metastasis classification of thymoma. Cancer. 1991;68:1984-7.
18. Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymoma with special reference to their clinical stages. Cancer. 1981;48:2485-92.
19. Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int. 1994;44:359-67.
20. Moran CA, Walsh G, Suster S, Kaiser L. Thymoma II. A clinicopathologic correlation of 250 cases with a proposed staging system with emphasis on pathologic assessment. Am J Clin Pathol. 2012;137:451-61.
21. Maggi G, Casadio C, Cavallo A, et al. Thymoma: results of 241 operated cases. Ann Thorac Surg. 1991;51:152-6.
22. Zhang H, Lu N, Wang M, et al. Postoperative radiotherapy for stage I thymoma: a prospective randomized trial in 29 cases. Chin Med J (Engl). 1999;112:136-8.
23. Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation, therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer. 2004;44:369-79.
24. Ridley GJ, Huang J. Induction therapy for locally advanced thymoma. J Thorac Oncol. 2010;
5:S323-26.
25. Venuta F, Rendina EA, Longo F, et al. Long-term outcome after multimodality treatment for stage III thymic tumors. Ann Thorac Surg. 2003;76:1866-72.
26. Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg. 2008;85:385-9.
27. Berruti A, Borasio P, Roncari A, et al. Neoadjuvant chemotherapy with Adriamycin, cisplatin, vincristine, and cyclophosphamide (ADOC) in invasive thymomas: results in six patients. Ann Oncol. 1993;4:429-31.
28. Giaccone G, Wilmink H, Paul MA, et al. Systemic treatment of malignant thymoma: a decade experience at a single institution. Am J Clin Oncol. 2006;29:336-44.
29. Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol. 2011;29:2060-5.
30. Macchiarini P, Chella A, Ducci F, et al. Neoadjuvant chemotherapy, surgery, and postoperative radiation therapy for invasive thymoma. Cancer. 1991;68:706-13.
31. Lucchi M, Ambrogi MC, Duranti L, et al. Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Ann Thorac Surg. 2005;79:
1840-4.
32. Rea F, Sartori F, Loy M, et al. Chemotherapy and operation for invasive thymoma. J Thorac Cardiovasc Surg. 1993;106:543-9.
33. Moran CA, Suster S. Thymomas with prominent hemorrhagic and necrotic changes: a clinicopathologic study of 25 cases. Am J Surg Pathol. 2001; 25:1086-90.