Can Rash Associated With HER1/EGFR Inhibition Be Used as a Marker of Treatment Outcome?
Rash is a class effect of HER1/epidermal growth factor receptor(EGFR)-targeted agents, and has occurred with high frequency and ina dose-dependent manner in clinical trials of these agents in cancerpatients. Analysis of phase II trials of erlotinib (Tarceva) in non–smallcelllung cancer, head and neck cancer, and ovarian cancer shows asignificant association between rash severity and objective tumor response.Rash severity was highly significantly associated with survivalin patients with non–small-cell lung cancer receiving erlotinib; mediansurvival in patients with no rash was 46.5 days, compared with257 days in those with grade 1 rash (P < .0001) and 597 days in thosewith grade 2/3 rash (P < .0001). Similarly, for the combined non–smallcelllung cancer, head and neck cancer, and ovarian cancer studies,median survival in patients with no rash was 103 days, compared with191 days in those with grade 1 rash (P = .0001) and 266 days in thosewith grade 2/3/4 rash (P = .0001). Similar findings have been madewith cetuximab (Erbitux) and in some settings with gefitinib (Iressa).The strong association of rash severity with response/survival suggeststhat rash may serve as a marker of response to erlotinib treatment andmay be used to guide treatment to obtain optimal response. Dosingerlotinib at the maximum tolerated dose, which is associated with morefrequent and more severe rash, may improve response rates and survivaldurations. Further study of the potentially important associationbetween rash and outcome of treatment with EGFR-targeted agents isneeded.
ABSTRACT: Rash is a class effect of HER1/epidermal growth factor receptor(EGFR)-targeted agents, and has occurred with high frequency and ina dose-dependent manner in clinical trials of these agents in cancerpatients. Analysis of phase II trials of erlotinib (Tarceva) in non-smallcelllung cancer, head and neck cancer, and ovarian cancer shows asignificant association between rash severity and objective tumor response.Rash severity was highly significantly associated with survivalin patients with non-small-cell lung cancer receiving erlotinib; mediansurvival in patients with no rash was 46.5 days, compared with257 days in those with grade 1 rash (P Erlotinib (Tarceva) is an orallyavailable, potent, highly specific,reversible inhibitor ofHER1/epidermal growth factor receptor(EGFR) tyrosine kinase. Phase IItrials have shown clinical activity oferlotinib in non-small-cell lung cancer,head and neck cancer, and ovariancancer, with a common toxicitybeing a skin rash that typically is acneiformin character and mild to moderatein severity. This skin rash is aclass effect of EGFR inhibitors. Accumulatingdata with erlotinib andother EGFR inhibitors suggest thatrash may be predictive of objectivetumor response and prolonged survivalin cancer patients.Skin Rash as a Toxicity ofHER1/EGFR InhibitionHER1/EGFR is expressed in theepidermis, sebaceous glands, and hairfollicle epithelium, and is recognizedto play a role in the normal differentiationand development of skin folliclesand keratinocytes. Studies in mousemodels including HER1/EGFRknockoutstudies, expression of dominantnegative HER1/EGFR mutants,and partial HER1/EGFR inhibitionshowed that absence or blocking of thereceptor produced hair, skin, and eyeabnormalities, including necrosis anddisappearance of hair follicles andpresence of inflammatory elements inskin.[1-4] Administration of erlotinibin the nude mouse model producedskin lesions consisting of diffuse,mild-to-moderate thickening of theepidermis, infiltration of mostly acuteinflammatory cells in the dermis, andformation of dry scabs on the eyes.[5]The current prevailing hypothesis forthe mechanism of skin toxicities as-
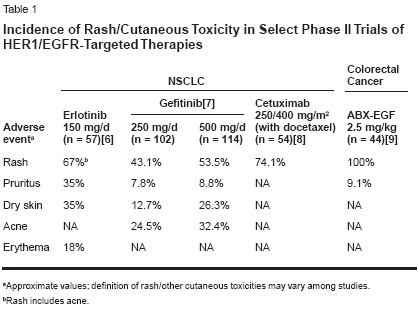
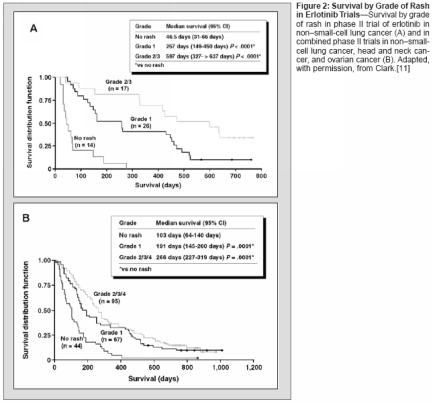
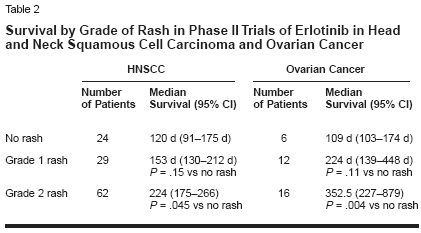
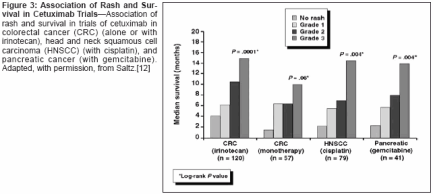
sociated with HER1/EGFR inhibitionis that this inhibition results in follicularocclusion due to lack of differentiationin the epithelium and affectssebaceous glands to produce a rosacea-like reaction, resulting in increasedproduction of inflammatorymediators and inflammation.The cutaneous toxicity associatedwith HER1/EGFR inhibition in preclinicalstudies is manifest primarilyas an acneiform skin rash in the clinicalsetting, with other skin disordersincluding pruritus, dry skin, anderythema. The skin rash is a class-relatedeffect, having been observed withall HER1/EGFR tyrosine kinase inhibitors(eg, erlotinib, gefitinib[Iressa]), dual HER inhibitors (eg,GW2016, and pan-HER inhibitors(eg, CI-1033), as well as with HER1/EGFR-specific monoclonal antibodies(eg, cetuximab [Erbitux], ABX-EGF,EMD72000). Table 1 provides an exampleof the consistently high rash/cutaneous toxicity frequency observedwith erlotinib, gefitinib, andcetuximab in phase II trials in non-small-cell lung cancer and with ABXEGFin a phase II trial in colorectalcancer[6-9]; it should be noted thatcutaneous toxicity is fairly infrequentin cancer chemotherapy, and thus categorizationand grading of the particularcutaneous toxicities have likelybeen somewhat inconsistent amongclinical trials. Available evidence indicatesthat the frequency of rash isdose-dependent.The rash generally occurs above thewaist, with involvement of the nosearea being most common, and is characterizedby clusters of monomorphicpustular lesions.[10] The most commonhistopathologic finding is neutrophilicinfiltration of dermal tissues.There is anecdotal evidence that rashspontaneously resolves in some patients.Currently, there are no clearguidelines for rash management.Treatment options include topical/systemicantibacterials (which may helpdue to superinfection of affected areas),retinoids, short-term systemiccorticosteroids (for inflammatory reaction),but such measures have generallyhad limited efficacy in resolvingrash. Further studies are requiredto improve understanding of rash etiologyand management.Association of Rash WithTumor Response and SurvivalObservations in studies with HER1/EGFR inhibitors have suggested thatrash may be predictive of tumor responseand prolonged survival andhave prompted analysis of these potentialassociations in study populations.The incidence and grade of rashin phase II studies of erlotinib in refractorynon-small-cell lung cancer,head and neck cancer, and ovariancancer are shown in Figure 1. A significantrelationship between rash severityand objective tumor responsewas observed in the trial in non-smallcelllung cancer and for the three studiescombined, with these data alsoshowing that objective responses werenot observed in any patients withoutrash (Figure 1).[11] Analysis of survivalin the trial in non-small-cell lungcancer shows a strong association ofrash with prolonged survival (Figure2). Median survival among patientswithout rash was 46.5 days, comparedwith 257 days in those with grade 1rash (P < .0001) and 597 days in thosewith grade 2/3 rash (P < .0001).As shown in Table 2, there weretrends toward significantly increasedsurvival in patients with grade 1 rashin the erlotinib phase II trials in headand neck cancer and ovarian cancer, andthe occurrence of grade 2 rash was associatedwith a significant increase insurvival duration in both studies. Poolingof the three phase II studies shows astrong association of rash with prolongedsurvival (Figure 2); median survivalin patients with no rash was 103days, compared with 191 days in thosewith grade 1 rash (P = .0001) and 266days in those with grade 2/3/4 rash(P = .0001).With regard to other EGFR-targetedagents, there is clear evidenceof an association of rash and survivalwith cetuximab. As shown in Figure3, a significant relationship betweenrash grade and survival has been observedin four studies of cetuximabalone or in combination in nearly 300patients with colorectal cancer, headand neck cancer, or pancreatic cancer.[12] Evidence of such an associationcurrently is somewhat less strongfor gefitinib, although limited data inthis regard have been reported; as yet,no data on the potential association ofrash with response/survival have beenreported from the IDEAL trials of thisagent in non-small-cell lung cancer.However, in the IDEAL trials, the incidenceof rash was slightly higher inpatients who received the higher doseof gefitinib (500 mg) as shown in Table1, and there was no difference in survivalin the patients who received 500or 250 mg. That said, an analysis ofthe relationship between rash and survivalwithin each group of patients hasnot been presented.In a phase II study of gefitinib in52 patients with head and neck cancer,skin toxicity was significantlyassociated with objective response(P = .004), progression-free survival(P = .0002), and overall survival(P = .001).[13] More recently, skinrash was reported to be associated withenhanced survial in a phase II studyof gefitinib in patients with bronchioalveolarcarcinoma.[14] However, nocorrelations between skin toxicity andresponse/survival were observed in aphase II study of gefitinib in combinationwith carboplatin (Paraplatin)/paclitaxel in 24 patients with non-small-cell lung cancer or in a phase IIstudy of gefitinib combined withFOLFOX 4 in 43 patients withcolorectal cancer.[15,16] It bears notingthat gefitinib added no benefit tocarboplatin/paclitaxel in the formerstudy and exhibited no antitumor activityin colorectal cancer in the latter.A relationship between rash and response/survival has been observedwith ABX-EGF, although data in thisregard are limited. No data on thesepotential associations have been reportedfor CI-1033 or EMD72000.There are important potential implicationsfor the association of rashand rash severity with response andsurvival. It is possible that rash can beused as a marker for clinical activity,and it may be that rash should be usedas a tool to maximize clinical response.The association of rash and survivalobserved with erlotinib, for example,suggests that this agent should begiven at its maximum tolerated dose,at which rash is more frequent andsevere, in order to achieve greater responserates and survival durations.These important implications are beingassessed in a phase II erlotinibdose-to-rash trial. In this trial, patientswith stage IIIB/IV non-small-cell lungcancer who have received at least oneprior chemotherapy regimen and whohave Eastern Cooperative OncologyGroup performance status of 0 or 1 arereceiving a starting dose of 150 mgdaily, with the dose being escalated in25- or 50-mg increments in the absenceof grade 2 or worse toxicity. Theprimary study outcome measures areobjective response rate and durationof response analyzed by grade of rash.
Correlation of Rash WithPharmacokinetic ParametersLimited available data suggest apotential correlation between rashand increased erlotinib exposure.Data from a phase II trial of erlotinibmonotherapy in patients withbreast cancer suggest a correlationbetween erlotinib area under theconcentration:time curve and maximumseverity of rash experienced(Figure 4).[16] There was a strongtrend toward reduced time to most severerash experienced in patients withdrug exposure above the 25th percentilecompared with those with lower
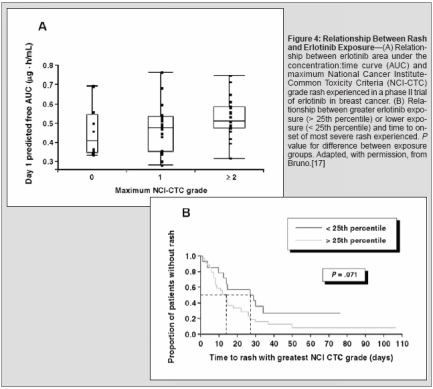
drug exposure (P = .071) (Figure 4);overall, the median time to rash onsetwas 8 days in the higher-exposuregroup compared with 14.5 days in thelower-exposure group and the mediantimes to highest grade of rash were 12days and 28 days, respectively. Thesepharmacodynamics observationscould be the result of pharmacokineticvariation or pharmacogenomic heterogeneityamong patients or both, but donot rule out the ability to increase rashfrequency or severity by increasing thedrug dose. These observations providesome support for the notion that increasingdrug exposure may result inmore severe rash and reduced time torash onset as an indicator of improvedclinical response and a predictor ofimproved outcome.ConclusionRash appears in most cases to be anecessary, but not sufficient, conditionfor response to erlotinib treatment. Theconsistent relationship between highergrades of rash and increased responserates or survival durations witherlotinib treatment suggests that rashmay be an important tool to optimizingtreatment outcomes. Further studiesand retrospective analyses of priorstudies are needed to improve understandingof this relationship.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Threadgill W, Dlugosz AA, Hansen LA,et al: Targeted disruption of mouse EGF receptor:effect of genetic background on mutantphenotype. Science 269:230-234, 1995.
2.
Sibilia M, Wagner EF: Strain-dependentepithelial defects in mice lacking the EGF receptor.Science 269:234-237, 1995.
3.
Murillas R, Larcher F, Conti CJ, et al:Expression of a dominant negative mutant ofepidermal growth factor receptor in the epidermisof transgenic mice elicits striking alterationsin hair follicle development and skinstructure. Embo J 14:5216-5223, 1995.
4.
Luetteke NC, Phillips HK, Qiu TH, et al:The mouse waved-2 phenotype results from apoint mutation in the EGF receptor tyrosinekinase. Genes Dev 8:399-413, 1994.
5.
Desai B, et al: (abstract 203). Eur J Cancer38:63, 2002.
6.
Pérez-Soler R, Chachoua A, HubermanM, et al: Final results from a phase II study oferlotinib (Tarceva) monotherapy in patientswith advanced non-small cell lung cancer followingfailure of platinum-based chemotherapy(abstract P-611). Lung Cancer41(suppl 2):S246, 2003.
7.
Baselga J, et al: abstract 481. ESMO2002.
8.
Kim ES, Mauer AM, Tran HT, et al: Aphase II study of cetuximab, an epidermalgrowth factor receptor (EGFR) blocking antibody,in combination with docetaxel in chemotherapyrefractory/resistant patients withadvanced non-small cell lung cancer: final report(abstract 2581). Proc Am Soc Clin Oncol22:642, 2003.
9.
Meropol NJ, Berlin J, Hecht JR, et al:Multicenter study of ABX-EGF monotherapyin patients with metastatic colorectal cancer(abstract 1026). Proc Am Soc Clin Oncol22:256, 2003.
10.
Hidalgo M, Siu LL, Nemunaitis J, et al:Phase I and pharmacologic study of OSI-774,an epidermal growth factor receptor tyrosine kinaseinhibitor, in patients with advanced solidmalignancies. J Clin Oncol 19:3267-3279, 2001.
11.
Clark GM, Pérez-Soler R, Siu L, et al:Rash severity is predictive of increased survivalwith erlotinib HCl (abstract 786). Proc Am SocClin Oncol 22:196, 2003.
12.
Saltz L, Kies M, Abbruzzese JL, et al: Thepresence and intensity of the cetuximab-inducedacne-like rash predicts increased survival in studiesacross multiple malignancies (abstract 817). ProcAm Soc Clin Oncol 22;204, 2003.
13.
Cohen EE, Rosen F, Stadler WM, et al:Phase II trial of ZD1839 in recurrent or metastaticsquamous cell carcinoma of the head andneck. J Clin Oncol 21:1980-1987, 2003.
14.
West HL, Franklin WA, Gumerlock P, etal: ZD1839 (Iressa) in advanced bronchioalveolarcarcinoma (BAC): A preliminary reportof SWOG S0126 (abstract O-187). LungCancer 41(suppl 2):S56, 2003.
15.
Miller VA, Johnson D, Heelan RT, et al:A pilot trial demonstrates the safety of ZD1839(Iressa), an oral epidermal growth factor receptortyrosine kinase inhibitor (EGFR-TKI), incombination with carboplatin (C) andpaclitaxel (P) in previously untreated advancednon-small cell lung cancer (NSCLC) (abstract1301). Proc Am Soc Clin Oncol 20:326a, 2001.
16.
Cho CD, Fisher GA, Halsey J, et al: Aphase II study of gefitinib in combination withFOLFOX-4 (IFOX) in patients withunresectable or metastatic colorectal cancer(abstract 1062). Proc Am Soc Clin Oncol22:265, 2003.
17.
Bruno R, Mass RD, Jones C, et al: Preliminarypopulation pharmacokinetics (PPK)and exposure-safety (E-S) relationships oferlotinib HCL in patients with metastatic breastcancer (MBC) (abstract 823). Proc Am Soc ClinOncol 22:205, 2003.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.