Identifying Predictive and Surrogate Markers of Erlotinib Antitumor Activity Other Than Rash
The identification of predictive or surrogate markers of response toHER1/epidermal growth factor receptor (EGFR) inhibitor treatmentwould permit selection of patients most likely to respond to such treatment.Markers could consist of tumor characteristics (eg, characteristicsof the receptor or downstream signaling molecules and determinantsof resistance) or host characteristics (eg, pharmacokinetic parametersand toxicities). The occurrence of rash may constitute a surrogatemarker of response to erlotinib (Tarceva) treatment in patientswith non–small-cell lung cancer and other cancers. The erlotinib markeridentification program has been designed to identify and investigateother candidate markers by analysis of a large number of clinicalsamples from patients enrolled in erlotinib trials in non–small-cell lungcancer, including the phase III TALENT and TRIBUTE trials oferlotinib combined with chemotherapy and the phase III BR.21 trial oferlotinib monotherapy in advanced non–small-cell lung cancer. Thisprogram should both contribute to understanding of the molecular biologyof HER1/EGFR inhibition and result in identification of potentialmarkers that can be evaluated in the clinical setting.
ABSTRACT: The identification of predictive or surrogate markers of response toHER1/epidermal growth factor receptor (EGFR) inhibitor treatmentwould permit selection of patients most likely to respond to such treatment.Markers could consist of tumor characteristics (eg, characteristicsof the receptor or downstream signaling molecules and determinantsof resistance) or host characteristics (eg, pharmacokinetic parametersand toxicities). The occurrence of rash may constitute a surrogatemarker of response to erlotinib (Tarceva) treatment in patientswith nonâsmall-cell lung cancer and other cancers. The erlotinib markeridentification program has been designed to identify and investigateother candidate markers by analysis of a large number of clinicalsamples from patients enrolled in erlotinib trials in nonâsmall-cell lungcancer, including the phase III TALENT and TRIBUTE trials oferlotinib combined with chemotherapy and the phase III BR.21 trial oferlotinib monotherapy in advanced nonâsmall-cell lung cancer. Thisprogram should both contribute to understanding of the molecular biologyof HER1/EGFR inhibition and result in identification of potentialmarkers that can be evaluated in the clinical setting.
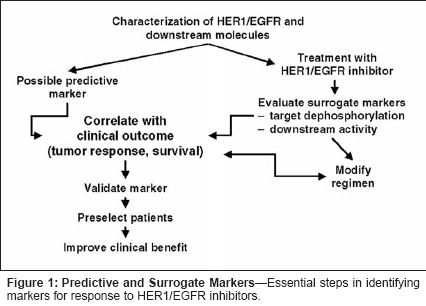
Erlotinib (Tarceva) belongs to anovel class of anticancer agentsthat inhibit the HER1/epidermalgrowth factor receptor (EGFR) tyrosinekinase. This agent has shownpromising activity in early-phaseevaluation in patients with non-smallcelllung cancer and is now beingevaluated in phase III trials. Identificationof a marker that could readilybe used to reliably predict response toerlotinib in non-small-cell lung cancerwould permit effective targeting oftreatment to those patients most likelyto respond. Identification of a surrogatemarker for antitumor activitywould also help to optimize clinicalbenefit of treatment. There are numerouschallenges inherent to identificationof such predictive and surrogatemarkers.Studies of Prognostic Factors inNon-Small-Cell Lung CancerPrognostic factors in non-smallcelllung cancer may offer insights intoidentification of predictors of response,particularly if the prognosticfactors can serve as targets of therapy.However, prognostic factors in adisease are not necessarily mark-ersthat predict response to treatment.Predictive markers can be specificto particular therapies, orcan be nonspecific for differenttypes of therapy. An obvious choicefor a marker that could predict responseto HER1/EGFR inhibitors isoverexpression of the receptor.However, analysis of receptor characteristicsby immunohistochemistry inpatients with non-small-cell lungcancer has generally indicated thatHER1/EGFR overexpression alone isnot a strong predictor of prognosis.[1]One study in patients undergoingsurgery showed that whereas highHER1/EGFR expression alone wasnot a strong prognostic indicator, thecombination of high HER1/EGFR andhigh HER2 expression was predictiveof poorer survival and the combinationof low HER1/EGFR and lowHER2 expression was predictive ofimproved survival; these results needto be confirmed in larger groups ofpatients.[2] Other studies using immunohistochemistryhave suggested thatHER1/EGFR expression is a strongpredictor of prognosis in head andneck, ovarian, cervical, and bladdercancers, and a moderate predictor ingastric, breast, colorectal, and endometrialcancers.[1] It should benoted, however, that the effort to evaluatethe prognostic utility of measuringreceptor expression is confoundedby problems with accurately assessingreceptor expression and the absence ofa standardized scoring system for levelsof expression. More important fromthe vantage point of desiring a predictivemarker for response to treatment,to date there is no evidence of a correlationbetween level of receptor expressionand clinical response with any receptorinhibitor.The complexity of identifying geneticprognostic features that mightserve as predictive markers is highlightedby two recent studies. Wigleet al analyzed mRNA of 3,000 genesusing microarray techniques to identifymarkers of non-small-cell lungcancer recurrence after surgery, andfound 16 genes that were prognosticindicators[3]; 15 of these, includingboth genes that were overexpressedand those that were underexpressed,demonstrated statistically significantdifferences in expression level betweengood and bad prognosis groups.The molecular prognostic factors didnot correlate with histologic subtype,and prognosis was independentHER1/EGFR expression. In anotherstudy by Sugita et al utilizing tissuefrom metastatic non-small-cell lungcancer, microarray analysis of 3,000genes yielded 20 genes (all testis-likeor melanoma-like) that discriminatedbetween good and bad prognosisgroups[4]; none of these geneswas identical to the 15 identified byWigle et al.Considerations in ClinicalEvaluation of TargetedTherapiesThere is no universally recognizedmeasurable biologic predictor ormarker of response to conventionalchemotherapy in non-small-cell lungcancer. Selection of regimens has beenbased on potency as indicated by objectiveresponse rates. It has been demonstratedthat combination therapy issuperior to monotherapy in terms ofsurvival advantage, but no combinationhas been shown to be superior toanother among accepted therapies.Against this background, a number ofmethodologic and statistical issuesplague evaluation of targeted therapiesin the clinical trial setting. Are targetedtherapies applicable to a whole populationof patients with the same histologicallyheterogeneous histology? Isit possible to demonstrate efficacy inthe conventional clinical trial settingwithout selecting patients based on thepresence of a specific therapeutic target?Is expression of a specific targetsufficient to warrant specific targetedtherapy?The potential impact of applying atargeted therapy to a population withhistologically defined disease can beappreciated by a hypothetical exampleinvolving the HER2 inhibitortrastuzumab (Herceptin), which is activein HER2-positive breast cancer.Based on findings in registration trialsin metastatic breast cancer, it canbe assumed that there is no disadvantagein using trastuzumab in HER2-negative patients and that survival inHER2-negative patients is 20 months.In a hypothetical clinical trial evaluatingstandard chemotherapy with orwithout trastuzumab in 750 patients ofwhom 30% have HER2-positive disease,overall survival would be 21.6months in trastuzumab-treated patientsand 20 months in standard therapypatients, based on existing data on survivalrates. This difference would notachieve statistical significance, leadingto the conclusion that trastuzumabhas no or little effectiveness in thissetting. Demonstration of a statisticallysignificant difference in survivalwould require a study population ofapproximately 2,500 patients on theassumption that 30% had HER2-positivedisease.Additional difficulties in evaluatingtargeted therapies are evident from areal-world study of trastuzumab inpatients with HER2-positive non-small-cell lung cancer.[5] In this study,cisplatin/gemcitabine (Gemzar) vscisplatin/gemcitabine/trastuzumabproduced response rates of 41% vs36%, median times to progression of7.2 vs 6.3 months, and median progression-free survival of 7.0 vs 6.1months. HER2 overexpression wasfound in only 20% of patients screenedusing immunohistochemistry. HER2gene amplification was detected inonly 2% of patients screened usingfluorescence in situ hybridization(FISH); tumor regression greater than50% occurred in 5 of 6 patients withHER2 gene amplification on FISHwho received trastuzumab. It can thusbe appreciated that precisely what constitutesa marker predictive of responsemay depend both on determining whatlevel of the marker is associated withspecific risk and on the availability oftechniques to accurately detect andmeasure that marker.Candidate Markers forHER1/EGFR InhibitorsHER1/EGFR signaling pathwaysconstitute a highly complex system.HER1 is responsible for activation ofa number of signaling pathways thatlead to cell proliferation. However,there are a number of other moleculesand receptors that could also be responsiblefor cell proliferation. Thus,HER1/EGFR tyrosine kinase activitycould be part of a system in which alarge number of different receptors/molecules are responsible for exponentialamplification of signaling pathwaysfor proliferation and other cellfunctions, or part of a system in whichactivation of a small number of kinasesdownstream from the receptor is responsiblefor proliferation and otherfunctions, perhaps through a clonalevent involving the receptor molecule.The latter situation is now known toobtain in the case of HER2 gene amplificationin metastatic breast cancer,increased BCR/ABL expression resultingfrom a translocation in chronicmyelogenous leukemia, and in a smallnumber of other settings.The essential steps in identifyingpotential predictive/surrogate markersfor response to HER1/EGFR inhibitorsare outlined in Figure 1. The initialstep is to characterize the receptorand the downstream molecules activatedby activation of the receptor. Theultimate test of the marker is that itaccurately predicts clinical response totreatment and provides a ready meansfor preselecting patients and optimizingtreatment benefit. Markers mayconsist of (1) tumor characteristics-eg, characteristics of the target receptoror downstream signaling moleculesor determinants of resistance to HER1/EGFR inhibition; or (2) patient characteristics-eg, pharmacokinetics andtoxicities.Tumor Characteristics
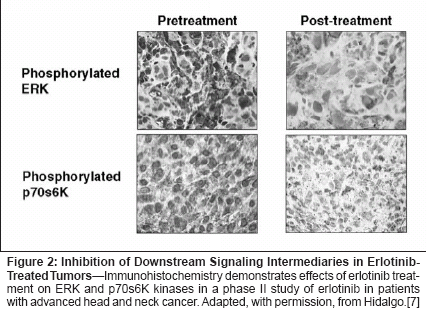
Target characteristics that couldconstitute potential markers includeconcentration or level of expression ofthe inactive receptor, or characteristicsof the activated receptor, includingbinding ligands, dimerization partners,mutations in receptor or downstreammolecules, and phosphorylation statusof downstream kinases. With regard topotential gene alterations, theEGFRvIII variant is associated with aconstitutively active (ligand-independent)tyrosine kinase, and is known tobe common in some central nervoussystem tumors; it has been reported tobe present in 15% to 30% of non-small-cell lung cancer tumors, but itsfrequency using high-precision methodsof detection and its significancein non-small-cell lung cancer remainlargely undefined. Little currently isknown about other HER1/EGFR mutationsor polymorphisms that mightaffect response to inhibitors.Erlotinib inhibits phosphorylationof HER1/EGFR in a dose-dependentmanner.[6] Recent investigations haveprovided evidence that erlotinib treatmentresults in dephosphorylation ofimportant downstream kinases ERKand p70s6K (Figure 2), indicating thatHER1/EGFR tyrosine kinase inhibitionis accompanied by reduced activationof signaling pathways.[7] It remains,unclear, however, to what degreethis inhibition is associated withclinical outcome in non-small-celllung cancer.
Determinants of acquired resistanceto HER1/EGFR inhibitors couldbe used to predict response to treatmentor guide dose modifications. Inan ongoing study, HN5 head and neckcancer cells were successively passagedin increasing concentrations oferlotinib, resulting in increasederlotinib 50% inhibitory concentrations.[8] This decrease in sus-ceptibilitywas accompanied bydownregulation of HER1, HER2, andHER3 receptors and by increased levelsof phosphorylated Akt kinase, indicatingthe activity of other potentiallyimportant signaling pathwaysdistinct from HER1/EGFR. Thesefindings thus far indicate that acquiredresistance to erlotinib in vitro is a progressivephenomenon associated withdownregulation of the HER family ofreceptors (possibly including selectionof rare cells with no/low HER expression)and upregulation of other genesinvolved in signaling. Translation ofsuch findings into implications for thein vivo setting requires further study.
Other recent data indicate the possibilityof innate resistance to HER1/EGFR inhibitors. Screening non-small-cell lung cancer patients fortreatment with the HER1/EGFR inhibitorgefitinib (Iressa) revealed anumber of genetic alterations and resultedin identification of GRG-(gefitinib resistance gene-1) as a candidategene associated with gefitinibresistance[9]; the gene encodes a cellsurfaceprotein closely associated withHER2. Initial analysis indicated thatGRG- expression was inversely associatedwith clinical response to treatment(P = .04). Clinical testing of thisand other candidate resistance genesis ongoing.Patient Characteristics
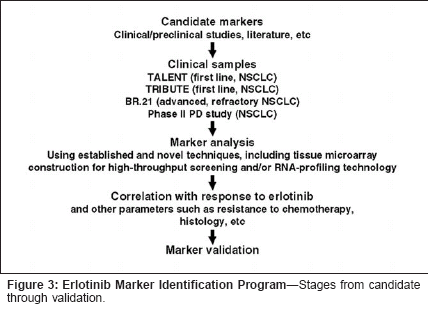
As noted, patient characteristicsthat may serve as predictive or surrogatemarkers include toxicities andhost pharmacokinetic characteristics.As reviewed by Prez-Soler elsewherein this supplement, there are accumulatingdata that rash may serve as asurrogate marker for effectiveness oferlotinib and other HER1/EGFR inhibitorsand could prove to be usefulin guiding drug dosage.With regard to pharmacokineticparameters, we have recently performeda study assessing the relationshipbetween plasma erlotinib steadystateconcentrations and survival inhead and neck cancer patients from aphase II trial of erlotinib.[10] Whenhazard ratios of survival are analyzedaccording to pharmacokinetic results,the median plasma concentrations ofboth erlotinib and OSI-420 at the 5-10 hour postdose window (hazard ratio1.054, P = .021 and hazard ratio1.422, P = .005, respectively), as wellas those of OSI-420 at trough window(20-25 hours) (hazard ratio 1.387,P = .0014), predict for improved overallsurvival. Analysis of rash in thisstudy population also showed a significantpredictive effect of rash forprolonged survival. We did not findany correlation between erlotinibplasma concentrations at steady stateand presence of rash, although thislatter finding may be due to the relativelysmall number of patients includedin the analysis.Other potential pharmacokineticvariables that require investigation toascertain potential effects on responseto HER1/EGFR inhibitor treatmentinclude cytochrome P450 polymorphisms,particularly involving the 3A4and 4A5 isoenzymes, and potentialpharmacokinetic interactions withchemotherapy or other concurrentlyadministered medications.Overall, more specific and powerfulcorrelative analyses for pharmacokineticfactors and adverse effects areneeded to identify potential predictive/surrogate markers.Erlotinib MarkerIdentification ProgramThe erlotinib marker identificationprogram has been designed to investigatethe potential utility of a largenumber of candidate markers in non-small-cell lung cancer (Figure 3). Tothis end, large numbers of clinicalsamples are being obtained from patientsenrolled in the phase III TALENTand TRIBUTE trials of erlotinibcombined with chemotherapy as firstlinetreatment of advanced non-smallcelllung cancer, the phase III BR.21trial of erlotinib monotherapy in advanced,refractory non-small-cell lungcancer, and a phase II pharmacodynamicsstudy in which biopsy samplesare being obtained prior to and duringerlotinib therapy with the primary intentof assessing effects of treatmenton signaling pathways. Candidatemarker analysis is to be performedusing standard and novel techniques,including reverse transcriptase polymerasechain reaction and tissuemicroarray construction for highthroughputscreening. It is hoped thatthis program will result in discoveryand validation of predictive/surrogatemarkers that will permit identificationof patients most likely to benefit fromerlotinib treatment.ConclusionNumerous opportunities exist toidentify candidate predictive and surrogatemarkers for response toerlotinib and other HER1/EGFR inhibitors,and development of novelmethods for marker analysis is ongoing.Further research is necessary tomore fully understand the componentsand interactions of the HER signalingpathways. Analysis of a large numberof clinical samples from non-smallcelllung cancer patients in erlotinibtrials in the erlotinib marker identification program will permit advancesin understanding the molecular biologyof response and nonresponse toHER1/EGFR inhibition and may resultin identification of markers thatwill allow optimal benefit to be gainedfrom treatment with erlotinib and othertargeted agents.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Nicholson RI, Gee JM, Harper ME: EGFRand cancer prognosis. Eur J Cancer 37(suppl4):S9-S15, 2001.
2.
Sridhar SS, Seymour L, Shepherd FA:Inhibitors of epidermal-growth-factor receptors:A review of clinical research with a focuson non-small-cell lung cancer. Lancet Oncol4:397-406, 2003.
4:397-406, 2003.
3.
Wigle DA, Jurisica I, Radulovich N, etal: Molecular profiling of non-small cell lungcancer and correlation with disease-free survival.Cancer Res 62:3005-3008, 2002.
4.
Sugita M, Geraci M, Gao B, et al: Combineduse of oligonucleotide and tissuemicroarrays identifies cancer/testis antigens asbiomarkers in lung carcinoma. Cancer Res62:3971-3979, 2002.
5.
Gatzemeier U, Groth G, Hirsh V, et al:Gemcitabine/cisplatin alone and withtrastuzumab (Herceptin) in patients with nonsmallcell lung cancer overexpressing HER2:Results of a randomized phase II study (abstract1185). Proc Am Soc Clin Oncol 21:297A, 2002.
6.
Moyer JD, Barbacci ED, Iwata KK, et al:Induction of apoptosis and cell cycle arrest byCP-358,774, an inhibitor of epidermal growthfactor receptor tyrosine kinase. Cancer Res57:4838-4848, 1997.
7.
Hidalgo M, Malik S, Rowinsky E, et al:Inhibition of the epidermal growth factor receptor(EGFR) by OSI-774, a specific EGFRinhibitor in malignant and normal tissues ofcancer patients (abstract 281). Proc Am SocClin Oncol 20:71a, 2001.
8.
Pérez-Soler R, Ling Y-H, Lia M, et al:Molecular mechanisms of resistance to theHER1/EGFR tyrosine kinase inhibitor erlotinibHCI in human cell lines (abstract 762). ProcAm Soc Clin Oncol 22:190, 2003.
9.
Natale RB, Shak S, Aronson N, et al:Quantitative gene expression in non-small celllung cancer from paraffin-embedded tissuespecimens: Predicting response to gefitinib, anEGFR kinase inhibitor (abstract 763). Proc AmSoc Clin Oncol 22:190, 2003.
10.
Soulières D, et al: J Clin Oncol (in press).