Imatinib Mesylate: A Molecularly Targeted Therapy for Gastrointestinal Stromal Tumors
Although their overall incidence is uncommon, gastrointestinal stromaltumors (GIST) are the most frequently encountered mesenchymaltumors of the GI tract. Their pathology has been recently defined bythe presence of KIT (transmembrane receptor tyrosine kinase). Themajority of GISTs have c-kit gain-of-function mutations mainly in exon11 (highly conserved juxtamembrane region) that eventuates in constitutiveactivation of KIT, promoting proliferation and antiapoptotic signaling.Imatinib mesylate (Gleevec) is a specific inhibitor of KIT kinaseactivation, and in phase II clinical trials has proven to be remarkablyefficacious in heavily pretreated GIST patients with advanced disease.The molecular and genomic determinants of response/resistancepatterns are the subject of ongoing studies, and adjuvant studies arealso under way. The initial evaluations of imatinib provide proof ofconcept for the hypothesis-driven design of selective molecularly targetedtherapies for solid tumor malignancies.
ABSTRACT: Although their overall incidence is uncommon, gastrointestinal stromal tumors (GIST) are the most frequently encountered mesenchymal tumors of the GI tract. Their pathology has been recently defined by the presence of KIT (transmembrane receptor tyrosine kinase). The majority of GISTs have c-kit gain-of-function mutations mainly in exon 11 (highly conserved juxtamembrane region) that eventuates in constitutive activation of KIT, promoting proliferation and antiapoptotic signaling. Imatinib mesylate (Gleevec) is a specific inhibitor of KIT kinase activation, and in phase II clinical trials has proven to be remarkably efficacious in heavily pretreated GIST patients with advanced disease. The molecular and genomic determinants of response/resistance patterns are the subject of ongoing studies, and adjuvant studies are also under way. The initial evaluations of imatinib provide proof of concept for the hypothesis-driven design of selective molecularly targeted therapies for solid tumor malignancies.
Gastrointestinal stromal tumors (GISTs) are the most commonly recognized mesenchymal tumors of the GI tract. Their true incidence is approximately 10 to 20 cases per 1 million, and yet, their defining characteristics and biology have remained largely obscured until recently.[1,2] Beginning with histogenetic findings by Mazur and Clark in 1983[3] and culminating with the discovery of the obligatory presence of dysregulated KIT protein, a much clearer definition of the pathobiology of this clinical entity has emerged.
The characterization of the molecular basis of this solid tumor has serendipitously coincided with the development of a small-molecule-specific targeted therapy and has provided a platform of discovery for rationally based hypothesis-driven clinical research. This review will outline the role of molecularly targeted therapies in the treatment of previously unresponsive solid tumors and will further elucidate how this bench-tobedside paradigm may provide a window into the future of cancer management.
Gastrointestinal Stromal Tumors
These unique stromal tumors of the GI tract have, in the past, been notably classified as spindle-cell nonepithelial smooth muscle tumors and were often grouped with leiomyosarcoma. However, careful examination by immunohistochemical (IHC) and ultrastructural analysis indicated that GISTs were distinct from other smooth muscle tumors and can have both myogenic and neurogenic features. This distinction was further elaborated by their similarity to the interstitial cells of Cajal (ICC), and it is now recognized that GIST cells and ICC cells are derived from a common progenitor or that perhaps the GIST cell is the neoplastic counterpart of the ICC cell.[4-6]
By consensus, it was recently established that GISTs constitute a separate clinical entity and that they are generally identified by the IHC expression of CD117, the antigen to a specific transmembrane epitope of KIT.[7] Equally paramount in understanding the pathobiology of GISTs was the initial finding that the noted overexpression of both membranebound and cytosolic KIT was associated with gain-of-function mutations in the c-kit oncogene.[8,9] In support of this conclusion, there are known rare inherited syndromes resulting in proband cases of multiple GISTs that appear to be associated with specific germ-line mutations in c-kit.[10,11]
The natural history of GISTs is often revealed by retrospective analysis and is somewhat speculative given that all stromal tumors of the GI tract were formerly classified as a single tumor type without distinction between a true GIST and a leiomyosarcoma. Several relatively large series have indicated a poor outcome for GIST patients even after complete resection,[12-14] with an approximate median survival of 60 months for the complete resection group. However, it is apparent that GIST patients with high-risk factors such as large tumor size (> 10 cm), nongastric origin, and a high mitotic rate will have a considerably shorter median survival. Even in GIST patients with more favorable characteristics, long-term disease-free survival is problematic. Patients presenting with metastatic disease or unresectable primary disease have a correspondingly shorter survival of generally less than 1 year.
Primary GISTs
Primary GISTs manifest most often in the stomach but can occur anywhere in the GI tract, with rare sporadic reports of peritoneal or omental primary GISTs. As with many GI tumors, particularly those of extramucosal origin, symptoms are insidious and can be associated with pain, weight loss, GI bleeding, or obstruction. Disease recurrence is almost invariably confined to the abdomen, with either multiple peritoneal sites, hepatic sites, or a combination of these.[15] Common combinations of cytotoxic chemotherapy and/or radiation therapy have been largely ineffective in the setting of recurrent, metastatic, or unresectable disease.[16]
Whether an initial primary GIST is benign or malignant may not be easily discernible by histopathologic examination and morphology. This is particularly relevant to the small incidental GISTs found during abdominal exploration. It is more likely that the disease represents a biologic continuum, with apparently benign GISTs noted to recur years after resection. Perhaps a better definition of a primary GIST with favorable characteristics would be a tumor with uncertain malignant potential.[17,18]
The mainstay of therapy for a malignant GIST is complete surgical resection. As previously noted, this has led to limited success, and until recently, no other therapeutic measures were available. Discovery of the molecular mechanisms of GISTs and insightful hypothesis-driven clinical research into the mechanisms of malignant transformation have resulted in a different approach to the treatment of many GIST patients, with an ongoing effort to enhance quality of life and prolong survival.
Role of c-kit in GISTs
The fundamental pathobiologic feature of the malignant phenotype of most GISTs is the dysregulated activation of the KIT signaling pathway. KIT is a transmembrane receptor tyrosine kinase encoded by the c-kit oncogene, the cellular homolog of v-kit (feline sarcoma virus). In nearly all GISTs, the KIT protein is constitutively activated, and thus, autophosphorylated.[ 19-22] This activation state is ligand-independent and results in dysregulated KIT kinase activity. In the majority of GISTs, noted in-frame gain-of-function mutations within c-kit eventuate in the phosphorylation of the KIT tyrosine residues providing docking sites for phosphotransferase activity, with resulting subsequent signaling transduction abnormalities favoring proliferation and enhancement of cell survival mechanisms.[23]
Imatinib Mesylate: An Active Small-Molecule Inhibitor of c-kit
FIGURE 1
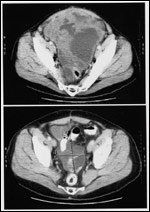
Pre- and Posttreatment CT Scans
Imatinib mesylate (Gleevec in the United States, Glivec in Europe) was initially designed as an orally administered inhibitor of the cytoplasmic fusion oncoprotein tyrosine kinase BCR/ABL and was successfully employed in clinical trials for chronic myelogenous leukemia.[24] Fortunately, this small-molecule inhibitor of receptor tyrosine kinase had broader pleotropic effects against structurally similar type 3 tyrosine kinases (including KIT, platelet-derived growth-factor receptor [PDGFR], and granulocyte- macrophage colony-stimulating factor [GM-CSF]).[25,26]
Preclinical in vitro studies have suggested that pharmacologic exploitation of the molecular signature of a GIST (dysregulated KIT) might present a viable therapeutic option for patients. These studies with imatinib in KIT-expressing malignant cell lines were notable for both antiproliferative and proapoptotic responses as well as a decrease in KIT tyrosine phosphorylation.[ 25] Based on these strong preclinical data, rational drug design, and knowledge of the clinical importance of KIT phosphorylation in the pathobiology of GIST, the first clinical application of imatinib resulted in a remarkable sustained response in a patient with a heavily pretreated metastatic GIST.[27]
Phase II/III Trials
This early study was quickly followed by a limited proof-of-principle randomized phase II trial to assess the safety and efficacy of two different doses of imatinib (400 and 600 mg) in patients with an unresectable or recurrent GIST expressing CD117. The study resulted in 147 patients receiving treatment, with 54%, 28%, and 14% demonstrating a partial but durable response, stable disease, and progressive disease, respectively.[28] These responses were remarkable considering these patients had been heavily pretreated and had not previously responded to any available therapy.
Based on data from this phase II trial, one GIST patient's response to imatinib is illustrated in Figure 1. This patient had a classic partial and durable response to therapy, as evidenced over a period of 6 months. The initial response data have been substantiated by a recent report with longer follow-up.[29]
Importantly, drug-related toxicity was not significant in the patients enrolled in this trial; indeed, any toxicity was quite manageable, as evidenced by the fact that 82% of patients remained on study with a median follow- up > 9 months. In addition, the study established that abrogation of tumor glycolytic activity as demonstrated by 18-fluorodeoxyglucose positron-emission tomography (PET) was an early and accurate predictor of response in these PET-avid tumors (Figure 2).[30] This type of PET response was seen in many cases even in the absence of typical regression on traditional cross-sectional imaging and was noted early in drug treatment.
FIGURE 2
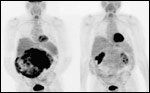
Pre- and Posttreatment PET Scans
Similar results were reported in a smaller trial conducted by the European Organization for Research and Treatment of Cancer (EORTC).[31] The importance of this molecularly targeted therapy is emphasized by the results of a recent EORTC study, which demonstrated a lack of imatinib activity in non-GIST sarcomas that did not express KIT.[32] An intergroup phase III study addressing the doseresponse question (400 vs 600 mg of imatinib daily) was recently completed, and the results of this study are pending.[33]
Response Assessment
The initial clinical experience with imatinib in GIST patients has provided valuable lessons in the assessment of response to an efficacious molecularly targeted drug, which can potentially be extrapolated to other solid tumor models and should serve as a paradigm as other kinase inhibitors are introduced into clinical research. Some of these principles are listed in Table 1. The standard methods of assessing response by measuring cross-sectional diameter may not accurately reflect the action of these small molecule inhibitors because their action may be more cytostatic than cytocidal, resulting in a change in tumor consistency (a myxoid appearance in the case of GIST) rather than size. This suggests that other imaging modalities (such as PET) that reflect functional tumor status can provide a more accurate picture of response and relapse.
TABLE 1

Early Trials of Imatinib Mesylate in GIST: Lessons Learned About Assessment of Response to Molecularly Targeted Drugs
Immediate relief of symptoms is quite characteristic of imatinib-treated GIST patients with large bulky disease. This factor, along with freedom from progression, may be a more significant response criterion than tumor regression, suggesting that classic metric diameter response measurements may continue to evolve over a long period of time or perhaps not be at all relevant to therapy with this evolving new class of drugs.
Adjuvant and Neoadjuvant Therapy
Another area of active clinical investigation of imatinib in GIST patients regards its efficacy in the setting of minimal microscopic residual disease after surgical resection or as a preoperative regimen to enhance resectability and long-term disease control. Observations from natural history data suggest that GIST patients with adverse characteristics such as large tumor size, high mitotic rate, and perforation have an exceedingly poor prognosis even after complete resection and should be candidates for trials of adjuvant therapy. The optimal dose and duration of imatinib in this setting is unknown but can be extrapolated from metastatic treatment trials.
Metastatic Disease Setting
Initial clinical experience with imatinib in metastatic disease suggested occasional mixed responses with areas of measurable tumor regressing and other areas not responding in the same patient. These mixed responses, as well as evidence of both intrinsic and acquired resistance, lead to the question of whether debulking surgery of marginally resectable primary or recurrent GISTs after induction therapy with imatinib can successfully extend disease-free survival or decrease the incidence of primary or secondary drug resistance. Surgical data prior to the availability of imatinib confirm a minimal role for resection in patients with a bulky or recurrent GIST.[34] However, it may now be time to revisit this relevant question.
Cooperative group clinical trials are under way to address the important issues regarding the use of this agent in these settings. The results of these adjuvant studies could establish a rationale for combining surgery with imatinib and perhaps for considering aggressive surgical debulking in this disease.
Factors Affecting Response and Resistance
Planned cooperative investigations into the molecular mechanisms of drug action were designed within the context of the initial phase II study, using both cell lines and fresh tumor specimens, These lines of inquiry have yielded important and novel information concerning tumor response and resistance. There appears to be a definite relationship between the presence and location of the gain-of-function mutation within the c-kit oncogene and response to imanitib (as well as subsequent clinical outcome).[35,36]
In the initial phase II trial, tumor tissue for evaluation of c-kit mutational status was available from 127 of the 147 patients, and 87% of these patients had mutations clustered within 4 exons (exons 9, 11, 13, 17), with the remaining 13% being wildtype for c-kit. The most common mutations in this group (85 patients) were found in exon 11 (juxtamembrane region of transmembrane KIT receptor) and predicted for a better response rate and survival than either exon 9 mutations or wild-type c-kit.[36]
Recently, 14 of 40 GIST tumors studied without an identifiable c-kit mutation have been shown to have PDGFR alpha-chain mutations in exonic sites that are structurally similar to the sites of mutations in c-kit.[37] These data suggest that some of the activating mutations, notably those outside of the juxtamembrane region in c-kit, interfere with drug binding and contribute to either primary or acquired resistance.
In most cases, the KIT signal transduction pathway appears to be critical to GIST cell survival, and although resistance is multifactorial, even in patients with a resistant phenotype, the majority maintain a similar pattern of phosphorylation downstream of the KIT kinase receptor [38]. This suggests that further manipulation of the KIT kinase pathway with combination drug therapy may be effective in overcoming this pattern of drug resistance. Work in this regard continues, and a small pilot study of another receptor tyrosine kinase inhibitor that affects the KIT pathway has shown efficacy in early results in imatinib-resistant GIST patients.[39]
Additional Molecular Mechanisms
Ongoing studies of genomic expression profiles in GIST specimens have provided further data on molecular mechanisms.[40,41] Global gene expression profiles from GIST specimens both before and during imatinib therapy and from GIST cell lines indicate selected response genes that are variably expressed when patients demonstrate a clinical response. Sprouty4A, a specific isoform of the Sprouty gene family involved in RAS regulation, is significantly and quantitatively downregulated in responsive tumors but is not altered in resistant tumors, providing an early and potentially clinically relevant polymerase chain reaction marker of response.[42] Additionally, a recent publication describing the development of a mouse model for GIST with an induced c-kit mutation will further investigations into the genetic molecular regulation of KIT in GIST and provide for an in vivo system that can be manipulated to evaluate molecular therapeutics.[43]
It is likely that future evaluations will provide sufficient additional data so that eventually GIST patients can be phenotyped prior to and just after imatinib therapy to determine whether they will respond to single-drug treatment with imatinib or whether alternative or combination therapies will be necessary to achieve maximal benefit. This will be accomplished without having to wait for a significant period of time and having to follow the patient by evaluating standard imaging criteria.
Conclusions
The rational hypothesis-driven application of a molecularly specific drug for GIST is a proof-of-principle example of how the expanding knowledge of genomics will lead to improved cancer therapies. This classic bench-tobedside approach to translational cancer research continues to resonate back to the bench for greater clarity and enhanced clinical relevance.
Financial Disclosure:The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Joensuu H, Fletcher C, Dimitrijevic S, etal: Management of malignant gastrointestinalstromal tumours. Lancet Oncol 3:655-664,2002.
2.
Miettinen M, Lasota J: Gastrointestinalstromal tumors-definition, clinical, histological,immunohistochemical, and molecular geneticfeatures and differential diagnosis.Virchows Arch 438:1-12, 2001.
3.
Mazur MT, Clark HB: Gastric stromaltumors: Reappraisal of histiogenesis. Am J SurgPathol 7:507-519, 1983.
4.
Kindblom LG, Remotti HE, AldenborgF, et al: Gastrointestinal pacemaker cell tumor(GIPACT): Gastrointestinal stromal tumorsshow phenotypic characteristics of the interstitialcells of Cajal. Am J Pathol 152:1259-1269, 1998.
5.
Erlandson RA, Klimstra DS, WoodruffJM: Subclassification of gastrointestinal stromaltumors based on evaluation by electronmicroscopy and immunohistochemistry.Ultrastruct Pathol 20:373-393, 1996.
6.
Miettinen M, Virolainen M, Maarit-Sarlomo-Rikala: Gastrointestinal stromal tumors-value of CD34 antigen in their identificationand separation from true leiomyomasand schwannomas. Am J Surg Pathol 19:207-216, 1995.
7.
Fletcher CD, Berman JJ, Corless C, et al:Diagnosis of gastrointestinal stromal tumors:A consensus approach. Hum Pathol 33:459-465, 2002.
8.
Hirota S, Isozaki K, Moriyama Y, et al:Gain-of-function mutations of c-kit in humangastrointestinal stromal tumors. Science279:577-580, 1998.
9.
Hirota S, Nishida T, Isozaki K, et al: Gainof-function mutation at the extracellular domainof KIT in gastrointestinal stromal tumors.J Pathol 193:505-510, 2001.
10.
Nishida T, Hirota S, Taniguchi M, et al:Familial gastrointestinal stromal tumors withgermline mutation of the KIT gene. Nat Genet19:323-324, 1998.
11.
Maeyama H, Hidaka E, Ota H, et al:Familial gastrointestinal stromal tumor withhyperpigmentation: Association with agermline mutation of the c-kit gene. Gastroenterology120:210-215, 2001.
12.
Dematteo RP, Lewis JJ, Leung D, et al:Two hundred gastrointestinal stromal tumors:Recurrence patterns and prognostic factors forsurvival. Ann Surg 231:51-58, 2000.
13.
Ng EH, Pollock RE, Munsell MF, et al:Prognostic factors influencing survival in gastrointestinalleiomyosarcomas. Implications forsurgical management and staging. Ann Surg215:68-77, 1992.
14.
Emory TS, Sobin LH, Lukes L, et al:Prognosis of gastrointestinal smooth muscle(stromal) tumors. Am J Surg Pathol 23:82-87,1999.
15.
Blanke CD, Heinrich MC, Eisenberg BL:Gastrointestinal stromal tumors. Curr TreatOptions Oncol 2:485-491, 2001.
16.
Edmonson J, Marks R, Buckner J, et al:Contrast of response to D-MAP plussargramostin between patients with advancedmalignant gastrointestinal stromal tumors andpatients with other advanced leiomyosarcomas(abstract). Proc Am Assoc Cancer Res 18:541a,1999.
17.
Corless C, McGreevey L, Haley A, et al:KIT mutations are common in incidental gastrointestinalstromal tumors one centimeter orless in size. Am J Pathol 160:1567-1572, 2002.
18.
Pidhorecky I, Cheney RT, Kraybill WG,et al: Gastrointestinal stromal tumors: Currentdiagnosis, biologic behavior, and management.Ann Surg Oncol 7:705-712, 2000.
19.
Eisenberg BL, von Mehren M: Pharmacotherapyof gastrointestinal stromal tumors.Expert Opin Pharmacother 4:869-874, 2003.
20.
Tuveson DA, Willis NA, Jacks T, et al:STI571 inactivation of the gastrointestinal stromaltumor c-KIT oncoprotein: Biological andclinical implications. Oncogene 20:5054-5058,2001.
21.
Rubin B, Singer S, Tsao C, et al: KITactivation is an ubiquitous feature of gastrointestinalstromal tumors. Cancer Res61:8118-8121, 2001.
22.
Kitamura Y, Hirota S, Nishida T: Molecularpathology of c-kit proto-oncogene anddevelopment of gastrointestinal stromal tumors.Ann Chir Gynaecol 87:282-286, 1998.
23.
Miettinen M, Majidi M, Lasota J: Pathologyand diagnostic criteria of gastrointestinalstromal tumors (GISTs): A review. Eur JCancer 38(suppl 5):S39-51, 2002.
24.
Druker BJ, Talpaz M, Resta DJ, et al:Efficacy and safety of a specific inhibition ofthe BCR-ABL tyrosine kinase in chronic myeloidleukemia. N Engl J Med 344:1031-1037,2001.
25.
Heinrich MC, Griffith DJ, Drucker BJ,et al: Inhibition of c-kit receptor tyrosine kinaseby STI 571, a selective tyrosine kinaseinhibitor. Blood 96:925-932, 2000.
26.
Joensuu H, Dimitrijevic S: Tyrosine kinaseinhibitor imatinib (STI571) as an anticanceragent for solid tumours. Ann Med 33:451-455, 2001.
27.
Joensuu H, Roberts PJ, Sarlomo-RikalaM, et al: Effect of the tyrosine kinase inhibitorSTI571 in a patient with metastatic gastrointestinalstromal tumor. N Engl J Med 344:1052-1056, 2001.
28.
Demetri GD, von Mehren M, Blanke CD,et al: Efficacy and safety of imatinib mesylatein advanced gastrointestinal stromal tumors. NEngl J Med 347:472-480, 2002.
29.
von Mehren M, Blanke C, Joensuu H, etal: High incidence of durable responses inducedby imatinib mesylate in patients withunresectable and metastatic gastrointestinalstromal tumor (abstract 1608). Proc Am SocClin Oncol 21:403a, 2002.
30.
van den Abbeele AD, Badawi RD: Useof positron emission tomography in oncologyand its potential role to assess response toimatinib mesylate therapy in gastrointestinalstromal tumors (GISTs). Eur J Cancer 38(suppl5):S60-S65, 2002
31.
van Oosterom AT, Judson I, Verweij J, etal: STI571, an active drug in metastatic gastrointestinalstromal tumors (GIST), an EORTCphase I study (abstract). Proc Am Soc ClinOncol 20:1a, 2001.
32.
Verweij J, van Oosterom A, Blay JY, etal: Imatinib mesylate is an active agent for gastrointestinalstromal tumors but does not yieldresponses in other soft-tissue sarcomas that areunselected for a molecular target. Eur J Cancer39:2006-2011, 2003.
33.
Benjamin R, Rankin C, Fletcher C, etal: Phase III dose-randomized study of Imatinibmesylate (IM) for GIST: Intergroup S0033 earlyresults (abstract 3271). Proc Am Soc Clin Oncol22:814, 2003.
34.
Mudan SS, Conlon KC, Woodruff JM,et al: Salvage surgery for patients with recurrentgastrointestinal sarcoma: Prognostic factorsto guide patient selection. Cancer 88:66-74, 2000.
35.
Heinrich MC, Corless CL, Blanke C, etal: KIT mutational status predicts clinical responseto STI571 in patients with metastaticgastrointestinal stromal tumours (GISTs) (abstract6). Proc Am Soc Clin Oncol 21:2a, 2002.
36.
Heinrich MC, Corless CL, von MehrenM, et al: PDGFRA and KIT mutations correlatewith the clinical responses to imatinibmesylate in patients with advanced gastrointestinalstromal tumors (GIST) (abstract 3274).Proc Am Soc Clin Oncol 22:815, 2003.
37.
Heinrich MC, Corless CL, Duensing A,et al: PDGFRA activating mutations in gastrointestinalstromal tumors. Science 299:708-710, 2003.
38.
Fletcher J, Corless C, Dimitrijevic S, etal: Mechanisms of resistance to imatinibmesylate (IM) in advanced gastrointestinal stromaltumor (GIST) (abstract 3275). Proc Am SocClin Oncol 22:815, 2003.
39.
Demetri G, George S, Heinrich MC, etal: Clinical activity and tolerability of the multitargetedtyrosine kinase inhibitor SU11248 inpatients (pts) with metastatic gastrointestinalstromal tumor (GIST) refractory to imatinibmesylate (abstract 3273). Proc Am Soc ClinOncol 22:814, 2003.
40.
Nielsen TO, West RB, Linn SC, et al:Molecular characterization of soft tissue tumors:A gene expression study. Lancet359:1301-1307, 2002.
41.
Allander SV, Nupponen NN, Ringner M,et al: Gastrointestinal stromal tumors with KITmutations exhibit a remarkably homogeneousgene expression profile. Cancer Res 61:8624-8628, 2001.
42.
Frolov A, Chahwan S, Ochs M, et al:Response markers and the molecular mechanismsof action of Gleevec in gastrointestinalstromal tumors (GISTs). Mol Cancer Ther9:3653-3654, 2003.
43.
Sommer G, Agosti V, Ehlers I, et al:Gastrointestinal stromal tumors in a mousemodel by targeted mutation of the KIT receptortyrosine kinase. Proc Natl Acad Sci100:6706-6711, 2003.