Chemotherapy Plus Irinotecan, Paclitaxel, Carboplatin Shows Activity
CHICAGO-Preliminary results of a phase I trial of induction chemotherapy followed by chemoradiotherapy with irinotecan (Camptosar), paclitaxel (Taxol), and carboplatin (Paraplatin) showed that this approach is feasible and active in stage III non-small-cell lung cancer (NSCLC), reported Ann M. Mauer, MD. The dose-limiting toxicity when combining these three drugs with concurrent chest radiotherapy was neutropenia, and weekly delivery of the regimen was not feasible at the originally planned doses. Dr. Mauer is assistant professor of medicine at the University of Chicago Pritzker School of Medicine in Chicago, Illinois.
CHICAGOPreliminary results of a phase I trial of induction chemotherapy followed by chemoradiotherapy with irinotecan (Camptosar), paclitaxel (Taxol), and carboplatin (Paraplatin) showed that this approach is feasible and active in stage III non-small-cell lung cancer (NSCLC), reported Ann M. Mauer, MD. The dose-limiting toxicity when combining these three drugs with concurrent chest radiotherapy was neutropenia, and weekly delivery of the regimen was not feasible at the originally planned doses. Dr. Mauer is assistant professor of medicine at the University of Chicago Pritzker School of Medicine in Chicago, Illinois.
"Both distant and locoregional treatment failures occur and contribute to mortality in stage III NSCLC. Consequently, combined-modality therapy with chemotherapy and radiation is now standard care. Newer agents have been incorporated into both induction chemotherapy and combined chemotherapy and radiotherapy regimens. The rationale for the combination used in our phase I trial is that carboplatin, paclitaxel, and irinotecan each demonstrates significant single-agent antitumor activity in NSCLC, that all three are radiosensitizers, and that they have non-overlapping toxicities," Dr. Mauer said.
Doses Had to Be Reduced
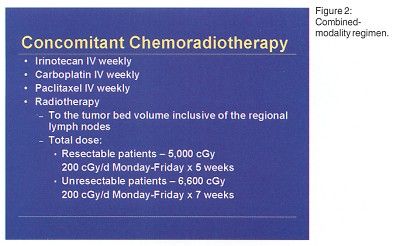
Twenty patients with histologically documented stage III NSCLC and no prior chemotherapy or chest radiotherapy were enrolled. The planned phase I treatment schema was induction chemotherapy with irinotecan/paclitaxel/carboplatin (Figure 1) followed by concomitant chemoradiotherapy with irinotecan/paclitaxel/carboplatin/radiation (Figure 2). "A third step of surgery, if feasible, was added because some patient became resectable as a result of chemoradiotherapy," Dr. Mauer said.
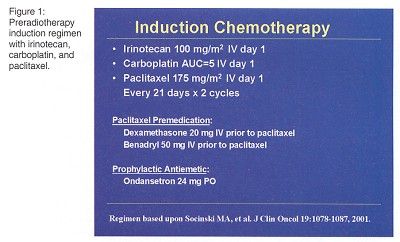
"We had planned to dose-escalate irinotecan but had to reduce doses due to toxicity," Dr. Mauer said.
Concomitant chemoradiotherapy at dose level I (irinotecan 30 mg/m²/wk, carboplatin area under the curve [AUC] 2/wk, paclitaxel 45 mg/m²/wk)produced grade 3 neutropenia in three patients. Two had treatment delayed because they met the criteria for dose-limiting toxicity due to failure to achieve white blood cell and neutrophil recovery to less than or equal to grade 1 toxicity on the day of scheduled chemotherapy. Treatment at dose level II (irinotecan 30 mg/m²/wk, paclitaxel 45 mg/m²/wk) produced dose-limiting grade 3 neutropenia in three of six patients treated. Treatment at dose level III (irinotecan 30 mg/m²/wk, paclitaxel 40 mg/m²/wk) produced dose-limiting neutropenia in one of six patients treated.
Nonhematologic toxicities during chemoradiotherapy included esophagitis: grade 3 esophagitis in four of 16 patients, grade 2 in six patients, and grade 1 in six patients. Grade 3 pneumonitis occurred in one patient and grade 2 pneumonitis in one patient, both at dose level II.
Range of Responses
Dr. Mauer reported that five patients (20%) had partial responses following induction chemotherapy and 14 had stable disease. Three patients developed distant metastatic disease during this portion of treatment. Nine of 15 patients had partial responses noted following chemoradiotherapy, and six patients had stable disease. Two patients developed distant disease progression during this stage of treatment.
Three patients had tumors that became resectable following chemoradiotherapy. Dr. Mauer said that one of the these patients had a complete pathologic remission. Two had partial remissions, one of whom later developed brain metastases.
A phase II study of this regimen is underway in stage III NSCLC to define the response activity and to establish the pattern of failure.