GVAX Autologous Vaccine Shows Activity in Lung Cancer
SAN FRANCISCO-GVAX, an autologous cancer vaccine, demonstrated antitumor activity in non-small-cell lung cancer (NSCLC) patients in a phase I/II multicenter clinical trial. Interim data were presented at the 37th Annual Meeting of the American Society of Clinical Oncology (ASCO abstract 1019).
SAN FRANCISCOGVAX, an autologous cancer vaccine, demonstrated antitumor activity in non-small-cell lung cancer (NSCLC) patients in a phase I/II multicenter clinical trial. Interim data were presented at the 37th Annual Meeting of the American Society of Clinical Oncology (ASCO abstract 1019).
Metastatic tumors disappeared completely in 3 of 22 patients who had previously failed radiation therapy and/or chemotherapy for advanced-stage lung cancer. Another patient had a tumor reduction of more than 50%, for an overall response rate of 18%. Two patients had a mixed response, and four had stable disease at a median follow-up of 5 months.
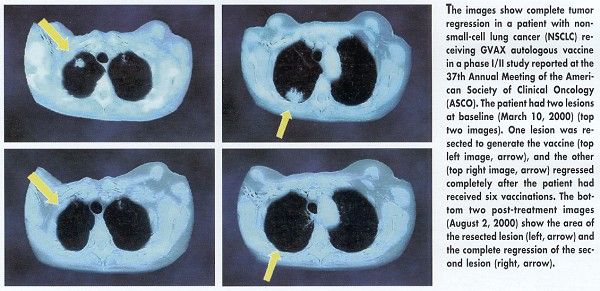
Among eight early-stage lung cancer patients who received the vaccine after surgery, seven were disease free at a median follow-up of 7 months. The eighth patient died.
"It was a fairly substantial response rate, which surprised us all," said Kristen Hege, MD, senior director of clinical research for Cell Genesys, Inc., Foster City, California. John Nemunaitis, MD, of US Oncology, Dallas, was lead author of the study, which was presented at a poster session.
GVAX has already met the main objectives of the ongoing trial, Dr. Hege said. It has been shown to be feasible as an antitumor agent, and, so far, no dose-limiting toxicities have been observed.
The most common adverse events were flu-like symptoms in some patients and a vaccine-site reaction observed in 92% of patients. "These were typical vaccine-site reactionsredness, itching, swellingsimilar to what you see with an insect bite," she said. "And that’s an encouraging side effect because it implies we are inducing an immune response at the vaccine site."
GVAX vaccines are also being evaluated for prostate cancer, myeloma, pancreatic cancer, and leukemia.
The vaccine is composed of tumor cells that are removed from the patient, irradiated, and genetically modified to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF). The vaccine, prepared overnight at the patient’s hospital, is designed to trigger an immune response specific to the patient’s own cancer cells.
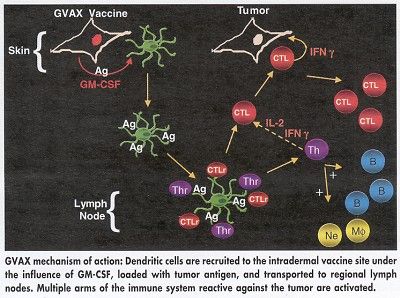
The GVAX vaccine used in the lung cancer trials was a custom-made product prepared from the patient’s own tumor cells. Cell Genesys has recently opened a new series of phase I/II trials with a centrally manufactured product that is subsequently mixed with the patient’s tumor cells. The centralized production process would be more viable commercially if and when GVAX gains approval for use. In either process, Dr. Hege stressed, the tumor cells are irradiated, so they will not grow when restored to the patient.
One concern was whether Cell Genesys could produce a sufficient yield of tumor cells for each patient. Again, Dr. Hege described the outcome as better than anticipated. The success rate was 77%, with a median cell dose of about 20 million cells per vaccination. "The patients were vaccinated every other week for 3 months, for a total of six vaccinations at a dose that ranged from 5 million cells to 100 million cells per vaccination," she said.
All told, 80 patients participated in the GVAX lung cancer trial reported at ASCO. Investigators will evaluate additional patients as they complete their vaccinations, and monitor all patients for long-term antitumor activity and delayed allergic response. Two patients in an earlier trial conducted by Glen Dranoff, MD, of Dana-Farber Cancer Institute, Boston, are reported to be in complete remission 3 years after receiving GVAX.