Investigators Wrestle With Reasons for Toxic Deaths in North American Colorectal Cancer Trials
COLORADO SPRINGS-Researchers from two large North American Intergroup colorectal cancer trials found an unexpectedly high rate of toxic deaths in trials containing irinotecan (Camptosar). No such problems had been apparently observed by oncologists using the combination of irinotecan, 5-fluorouracil (5-FU), and leucovorin (LV) (IFL) in routine clinical practice, and no such problems had been identified in the pivotal registration and European studies.
COLORADO SPRINGSResearchers from two large North American Intergroup colorectal cancer trials found an unexpectedly high rate of toxic deaths in trials containing irinotecan (Camptosar). No such problems had been apparently observed by oncologists using the combination of irinotecan, 5-fluorouracil (5-FU), and leucovorin (LV) (IFL) in routine clinical practice, and no such problems had been identified in the pivotal registration and European studies.
After the higher than expected rates of early death were reported in April, accrual to both North American studies was halted until the toxicity data could be analyzed. Researchers from the North American and European trials, as well as medical staff from Pharmacia Oncology, which manufactures irinotecan, discussed the unanticipated toxicity problems on July 26, 2001, at the University of Texas M. D. Anderson Cancer Center Investigators’ Workshop in Colorado Springs.
Possible Explanations
These experts advised clinicians to be vigilant in treating diarrhea in patients on IFL and to view the combination of diarrhea and neutropenia as a red flag for increased risk of serious complications.
The North American Intergroup trials used bolus 5-FU in combination with irinotecan and LV. Infusional fluorouracil, which is associated with a generally lower incidence of neutropenia and diarrhea, is more commonly used in Europe.
Leonard Saltz, MD, and colleagues demonstrated last year that a schedule of irinotecan, plus bolus 5-FU, and leucovorin was superior to the widely used regimen of 5-FU/LV in terms of objective tumor response, progression-free survival, and overall survival for patients with metastatic colorectal cancer. This led to Food and Drug Administration (FDA) approval of IFL for initial treatment of colorectal cancer, and IFL soon became widely accepted as the standard of care.
The IFL combination moved into North American Intergroup trials in comparison to oxaliplatin/5-FU/leucovorin and oxaliplatin/irinotecan in metastatic disease (study N9741) and in comparison to 5-FU/leucovorin as adjuvant treatment for patients with resected stage III disease (study C89803). Irinotecan is also being studied in Europe on an infusional schedule of irinotecan with 5-FU plus folinic acid vs 5-FU/folinic acid alone as adjuvant treatment for resected stage III colon cancer.
Current Status of North American Trials
C89803 adjuvant trial
Dr. Saltz, who is associate attending physician in the Gastrointestinal Oncology Service, Memorial Sloan-Kettering Cancer Center, New York City, discussed toxicity data from both North American studies. The adjuvant therapy trial, C89803 originally randomized patients with resected stage III colon cancer to five cycles of irinotecan (125 mg/m²/wk × 4 wks, q 6 wks), 5-FU (500 mg/m²/wk × 4 wks, q 6 wks), and leucovorin (20 mg/m²/wk × 4 wks, q 6 wks) or to four cycles of the Roswell Park regimen of 5-FU (500 mg/m²/wk × 6 wks, q 8 wks) and leucovorin (500 mg/m²/wk × 6 wks, q 8 wks). The accrual goal was 1,260 patients, and the study had enrolled 1,264 patients when accrual was halted in April 2001.
North Central Cancer Treatment Group (NCCTG) advanced CRC trial (N9741)
By the time C89803 was closed to accrual, the N9741 trial of irinotecan, 5-FU, and leucovorin as first-line treatment for advanced colorectal cancer had become quite complex. The original design of the NCCTG/Intergroup N9741 study is shown below.
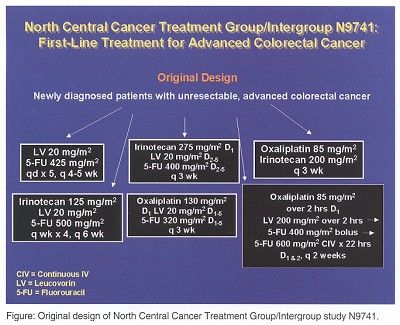
The original goals of N9741 were to compare time to tumor progression of each of the five experimental arms to the Mayo Clinic 5-FU/leucovorin control arm and to evaluate the survival rate, response rate, quality of life, and toxicity of each arm. This design was further changed in March of 2000 after the FDA approved IFL as first-line therapy for advanced colorectal cancer.
"Irinotecan plus bolus 5-FU and leucovorin became the ‘standard treatment arm’ against which regimens were compared in N9741," Dr. Saltz said. "At that point the Mayo Clinic 5-FU/leucovorin regimen was dropped from the trial."
In March 2000, real-time toxicity monitoring identified unacceptable life threatening (grade 4) and lethal (grade 5) toxicity rates in two of the study arms (oxaliplatin/Mayo 5-FU and irinotecan/Mayo 5-FU). At that point the study was redesigned to compare IFL to oxaliplatin/infusional 5-FU and to oxaliplatin/irinotecan. In April 2001 real-time toxicity monitoring again identified unacceptably high toxic deaths in the control arm (irinotecan/bolus 5-FU/leucovorin), and accrual was suspended.
Erroneous Comparison
Dr. Saltz said that initial efforts to understand the toxicity data were complicated by an erroneous comparison between the early death rate in these two studies and data from his previous study of 5-FU/leucovorin with and without irinotecan (study 0038).
"In both N9741 and C89803 trials, there was a strong trend toward higher early death rates in the irinotecan/5-FU/leucovorin arms than in the comparator arms," Dr. Saltz said. The real-time toxicity monitoring identified 60-day mortality from start of treatment as 4.8% and 2.2% for IFL on N9741 and C89803, respectively. However, deaths reported in study 0038 were investigator adjudicated toxic deaths, not all deaths within 60 days of starting treatment. When deaths within 60 days were examined in the 0038 study, the 60-day all-cause mortality was 6.7%, which was comparable to the 7.3% 60-day all-cause mortality in the Mayo 5-FU/LV control arm in that study. This highlights the importance of doing randomized controlled studies and the risk of drawing conclusions from nonrandomized data."
Dr. Saltz said that the death rates within 60 days of the first dose in the 0038 study showed no difference among the three treatment arms: irinotecan alone, irinotecan plus 5-FU and leucovorin, and 5-FU/leucovorin. Incidences of nonlethal toxicities for the IFL regimen in 0038, N9741, and C89803 appear to be similar.
Ominous Sign
"We did find that the appearance of diarrhea plus neutropenia is an ominous sign and something we need to take very seriously, perhaps including intervention with oral quinolones. With a toxic diarrhea, more bacterial contamination may be introduced into the circulation. A debilitated ability to mount a white blood cell response might then put the patient at increased risk of overwhelming sepsis," Dr. Saltz said.
"Irinotecan-based treatment should not be given if diarrhea has occurred within 24 hours before the planned treatment. A small proportion of patients will also show clear signs of toxicity after only one dose of treatment and will require dosage modifications."
North American investigators are considering more aggressive dose modification for toxicity as well as evaluating alternative dose and schedules in prospective clinical trials.
European Studies
European studies of irinotecan-based regimens include two ongoing colon cancer trials (V307/PETTAC 3 and ACCORD 2), two planned colon cancer trials (EORTC/PETTAC4 and QUASAR), and one ongoing rectal cancer study (AERO R98). These trials were described by Jean-Yves Douillard, MD, PhD, professor of medical oncology, at Centre Rene Gauducheau in Saint-Herblain, France.
Trial V307 for patients with stage II/III colon cancer is a multicenter, phase III, open-label randomized, adjuvant therapy trial comparing irinotecan in combination with a 5-FU/folinic acid infusional regimen to 5-FU/folinic acid without irinotecan. Patients are assigned to either weekly or biweekly regimens. The primary endpoint is disease-free survival (DFS) at 3 years in stage III patients. Secondary endpoints include DFS at 3 and 5 years, and overall survival (OS) at 7 years for stage II patients, and DFS and OS at 5 years for stage III patients.
The weekly regimen (AIO) utilized infusional 5-FU (Douillard et al, Lancet 355:1041-1047, 2000) and includes irinotecan at 80 mg/m², folinic acid at 500 mg/m² given intravenously over 2 hours, and 5-FU at 2,000 mg/m² given IV over 24 hours. The control arm uses the same regimen without irinotecan and with 5-FU at 2,600 mg/m². Treatment is on days 1, 8, 19, 22, 29, and 35. One cycle includes 6 infusions over 7 weeks.
The biweekly regimen is the unmodified DeGramont regimen (irinotecan 180 mg/m² on day 1, and folinic acid (FA) at 200 mg/m² given IV over 2 hours on days 1 and 2, plus 5-FU as a 400 mg/m² bolus and 5-FU as a 22 hour infusion at 600 mg/m² on days 1 and 2. The control arm is 5-FU/FA at the same doses without irinotecan. A complete cycle of 3 infusions requires 6 weeks.
Dr. Douillard said that 1,525 of the expected 1,800 patients have been enrolled. "There have been five deaths among the 1,525 patients treated so far," he reported. "These include one cardiorespiratory failure in a patient with pre-existing myocardiopathy, one stroke, one sudden death, one pulmonary embolism, and one occlusion. Deaths were equally balanced between the irinotecan and nonirinotecan arms, and none are considered related to treatment."
The Concerted Action on Colorectal Cancer trial #2 (ACCORD-2) is a phase III randomized trial of adjuvant treatment of resected high-risk colon cancer. The experimental regimen in this trial is the original DeGramont schedule of bimonthly infusional 5-FU/folinic acid plus irinotecan. The control regimen is 5-FU/FA without irinotecan. The protocol calls for accrual of 400 patients, and present enrollment is 268. Dr. Douillard said that interim safety analysis of the first 200 patients treated revealed no unexpected toxicities or toxic deaths.
The European Association for Research in Oncology rectal cancer study (AERO R98) is a randomized, phase III trial comparing 5-FU/leucovorin to 5-FU/leucovorin plus irinotecan in curatively resected stage II and stage III rectal cancer patients. The control arm is either bimonthly infusional 5-FU/leucovorin or the Mayo Clinic regimen. The experimental arm is the infusional bimonthly regimen plus irinotecan. Dr. Douillard said that only 133 of the needed 600 patients have been enrolled and that interim safety analysis found no unexpected toxicities or toxic deaths.
Early Recognition
The number of toxic deaths in the North American cooperative group trials and the absence of such problems in European studies and in routine clinical practice have been areas of major concern for Pharmacia, which markets irinotecan. David Emanuel, MD, from the company’s clinical development department, said that analysis of all deaths occurring in the North American Intergroup studies resulted in the following advice for clinicians and researchers working with the bolus IFL regimen.
"Measures to prevent treatment-related deaths should include early recognition of and early intervention for the gastrointestinal syndrome of diarrhea, nausea, vomiting, cramping, and neutropenia. There should be earlier, more intensive supportive intervention on cycle 1 of treatment, including prophylactic and/or therapeutic use of oral quinolones or other antibiotics with broad gram-negative coverage, and prompt, aggressive management of diarrhea," Dr. Emanuel said.
The Pharmacia analysis of toxicity data also concluded that careful patient selection is essential, since increased risk of death was associated with PS of 2 or greater, age over 75 years, ascites, pleural effusion, malnutrition (baseline serum albumin < 3 g), and recent infection that had required antibiotic treatment.
Final Report Issued
A final report on mortality associated with IFL has been prepared by an external review board. The report is available on the Journal of Clinical Oncology (JCO) online and will be published in JCO on September 15.
Dr. Emanuel said that initiatives under consideration include more work using infusional regimens and follow-up studies seeking pharmacogenomic markers for patients at increased risk of toxicity.
Dr. Emanuel said that Pharmacia has received and reviewed 38 of the 43 charts for patients who died within 60 days of treatment initiation in the two North American cooperative group studies, which were conducted under the auspices of the NCCTG and of Cancer and Leukemia Group B (CALGB). The company’s ongoing analysis included review of available charts for 21 of the 23 patients who died in N9741 and available charts for 17 of the 20 patients who died in C89803.
The main area of concern was deaths occurring in 30 days or less from the beginning of treatment. Dr. Emanuel said that 12 of the 23 deaths in N9741 patients were in this period, and 9 deaths were greater than 30 days. In the C89803 patients, eight of the 20 deaths were within 30 days, and nine were after 30 days.
Deaths that had occurred within 60 days were classified as related, unrelated, or possibly related to treatment. Dr. Emanuel said that nine of 21 deaths on N9741 study and 11 of 17 deaths on C89803 study were classified as treatment-related by Pharmacia analysts. Five more deaths on N9741 and five more on C89803 were classified as possibly treatment related.
Must Educate Patient
The contrast between the toxic deaths seen in the North American Intergroup studies and the lack of such problems in the European studies sparked lively discussion. Dr. Douillard suggested that differences in supportive care might be one contributing factor.
"You must educate the patient about the potential toxicities related to these treatments," Dr. Douillard said. "Prophylaxis for the diarrhea does not work, so the patient must be prepared to begin treatment quickly. In Nantes, patients are provided with loperamide (Imodium) and told to take a dose after each liquid stool. If diarrhea persists after 48 hours of loperamide, the patient has a prescription for a quinolone antibiotic and knows to begin taking it and to report to the clinic," he continued.
"At our center, all treatment on these trials is delivered at home by a specially trained nurse. The patient is seen every 2 weeks by the physician, who reviews any problems and supplies the prescriptions for the next 2 weeks. We really do not see the levels of toxicity reported in the North American Intergroup studies, and I think that this may be why."
Community Experience
Speaking to the fact that similar problems with toxic deaths have not been seen in the community setting with irinotecan, Dr. Saltz suggested that the difference might be in part a result of investigators allowing protocol guidelines to override their clinical judgment.
Daniel G. Haller, MD, who co-chaired this session, agreed. "I don’t see a lot of toxicity with the irinotecan/5-FU/leucovorin regimen in my usual clinical practice. When I started entering patients on study, sometimes on weeks 3 and 4, I would look at the protocol and decide that, based on my clinical judgment, I really did not want to give the dose as scheduled," Dr. Haller said.
"As clinicians, we may find that some protocols are a little tight on weeks 3 and 4," he continued. "When we put patients on study, we are all afraid of the big bears coming in and citing us for a major protocol violation in their retrospective evaluation of treatment. The protocol might suggest to treat if the patient is PS 2, but that might include a ‘PS 2.8’ patient who should not be treated on that schedule off trial. If the way the protocol is written nudges you toward treatment but you would rather go the other way, document what you do and why, in your clinical judgment, the change is appropriate for the patient." Dr. Haller is professor of medicine at the University of Pennsylvania Cancer Center in Philadelphia.
"We need to see the patient every week and to ask not only, ‘How are you now?’ but also ‘How were you last weekend?’", said Dr. Saltz. "Patients want to please and will sometimes not tell you about diarrhea or other problems that started a few days ago but are now better," Dr. Saltz added.
He emphasized that an important point is whether the patient sees a physician every week until a cycle is completed with acceptable toxicity. "What we may be seeing is clinical toxicity followed by a crash because the toxicity was not adequately investigated."
Jaffer Ajani, MD, who co-chaired the session, recommended that monitoring of chemotherapy patients include use of a daily calendar on which the patient marks toxicity. "We have devised a patient calendar with the National Cancer Institute (NCI) toxicity criteria that might be expected with each combination of drugs," he said." If more description is necessary for the patients to understand, we provide it. The patients mark the calendar every day and bring it when they come back to the clinic. We find this very useful and are now trying to automate the analysis of these forms. This is a much more accurate way of assessing toxicity than waiting until the patients complete the first 4 weeks of a cycle and then asking them to look back and remember how many times they had a problem," Dr. Ajani said. He is professor of medicine at the University of Texas M. D. Anderson Cancer Center in Houston.