‘Dealer’s Choice’ Rectal Cancer Trial Proposed
CHICAGO-A "dealer’s choice" rectal cancer trial in which treatment would be chosen by the physician and patient together has been proposed by the National Cancer Institute (NCI) Gastrointestinal Intestinal Intergroup. The Intergroup now includes research groups from the American College of Surgeons Oncology Group, Cancer and Leukemia Group B, the Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, the NCI Canada, the National Surgical Adjuvant Breast and Bowel Project (NSABP), the Radiation Therapy Oncology Group, and the Southwest Oncology Group.
CHICAGOA "dealer’s choice" rectal cancer trial in which treatment would be chosen by the physician and patient together has been proposed by the National Cancer Institute (NCI) Gastrointestinal Intestinal Intergroup. The Intergroup now includes research groups from the American College of Surgeons Oncology Group, Cancer and Leukemia Group B, the Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, the NCI Canada, the National Surgical Adjuvant Breast and Bowel Project (NSABP), the Radiation Therapy Oncology Group, and the Southwest Oncology Group.
Al B. Benson III, MD, professor of medicine and director of the Clinical Investigations Program at Northwestern University’s Robert H. Lurie Comprehensive Cancer Center in Chicago, Illinois, discussed the proposed study. "This group has now come together with the goal to work as a united front for the first time, to design large trials for rectal cancer," Dr. Benson said.
The first Intergroup trial supported the use of continuous infusion 5-fluorouracil (5-FU) in rectal cancer. The second Intergroup trial (INT-0114) is raising significant concern because of deaths occurring beyond 5 years after treatment, especially in poor-risk patients. There were no significant differences in outcome by treatment arm among the nearly 1,700 patients on this study, with median follow-up of 7.4 years.
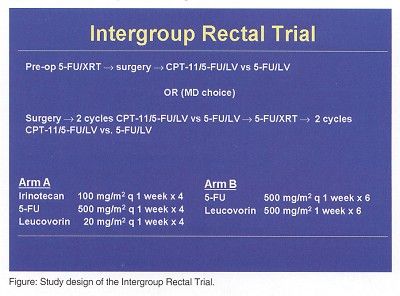
Area of Great Concern
One additional area of great concern is that for N0 patients, outcome was strongly dependent on the total number of nodes examined by the pathologist. Dr. Benson said this suggested that many patients who are classified as N0 are really N1. Patients thought to be node-negative who had fewer than 14 nodes examined had significantly worse survival than node-negative patients who had 14 or more nodes examined. This difference did not affect node-positive patients.
"Although this could be due to the surgeon not dissecting nodes adequately, it is more likely a sign of inadequate detection by the pathologist. The point has been raised by Intergroup pathologists that we need to start integrating much more careful attention to pathology and surgical issues in our clinical trial designs. We also need to start reimbursing pathologists and surgeons who are participating in these trials. Presently, most grants go to support medical oncology and the research offices," Dr. Benson said.
Dr. Benson said that one of the great disappointments in rectal research in the United States has been lack of success in conducting trials to compare preoperative vs postoperative therapy. Incomplete and preliminary data from the NSABP-R03 study suggests that preoperative therapy permits sphincter-saving surgery for more patients, although it does cause more toxicity.
"In Intergroup discussions there has been a dilemma about what to do. Do we focus on preoperative trial designs? Do we focus on postoperative trial designs? Or do we put a little bit of reality into what we are trying to accomplish? Consequently, the protocol that is being considered by the NCI Intergroup now is a dealer’s choice approach, similar to trial designs in Europe. The physician and patient will decide whether preoperative or postoperative radiation will be used. Radiation and chemotherapy options will include a couple of 5-FU based regimens. The question is: What is the role of irinotecan-based therapy compared to 5-FU/leucovorin?" Dr. Benson said.